Abstract
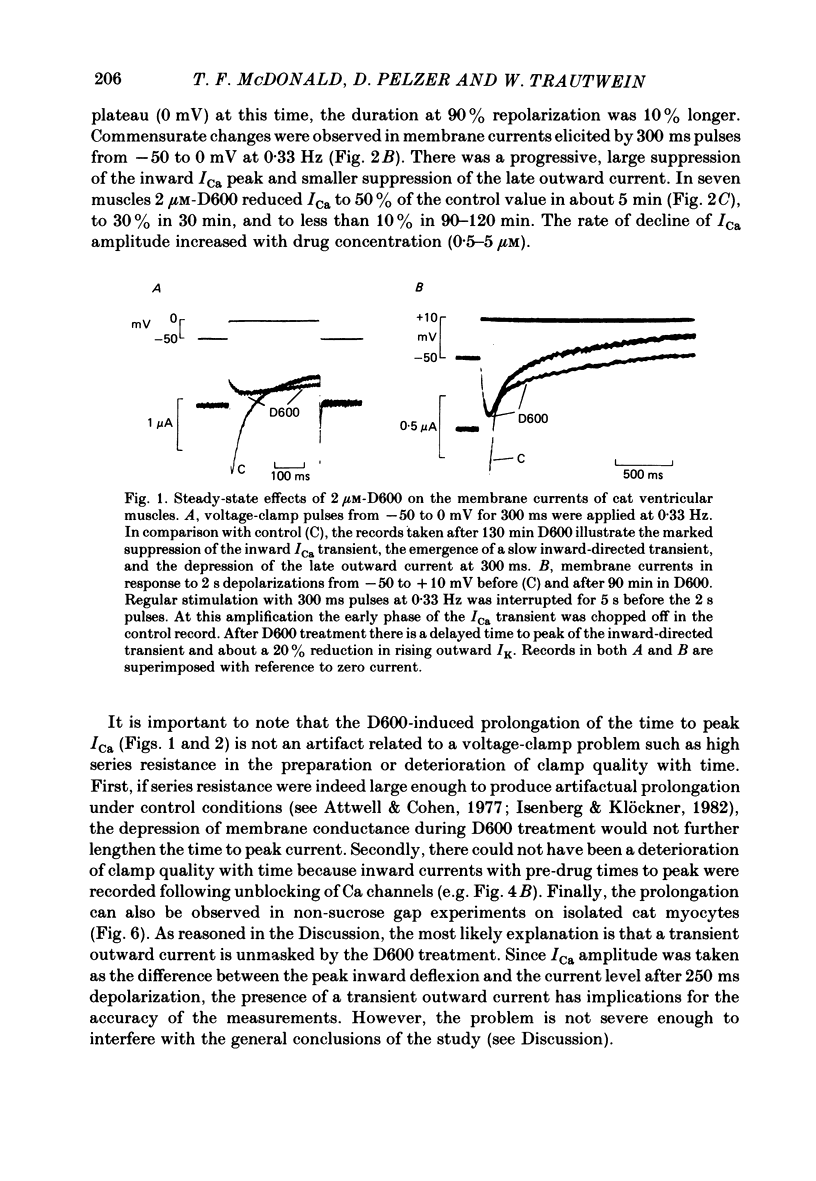
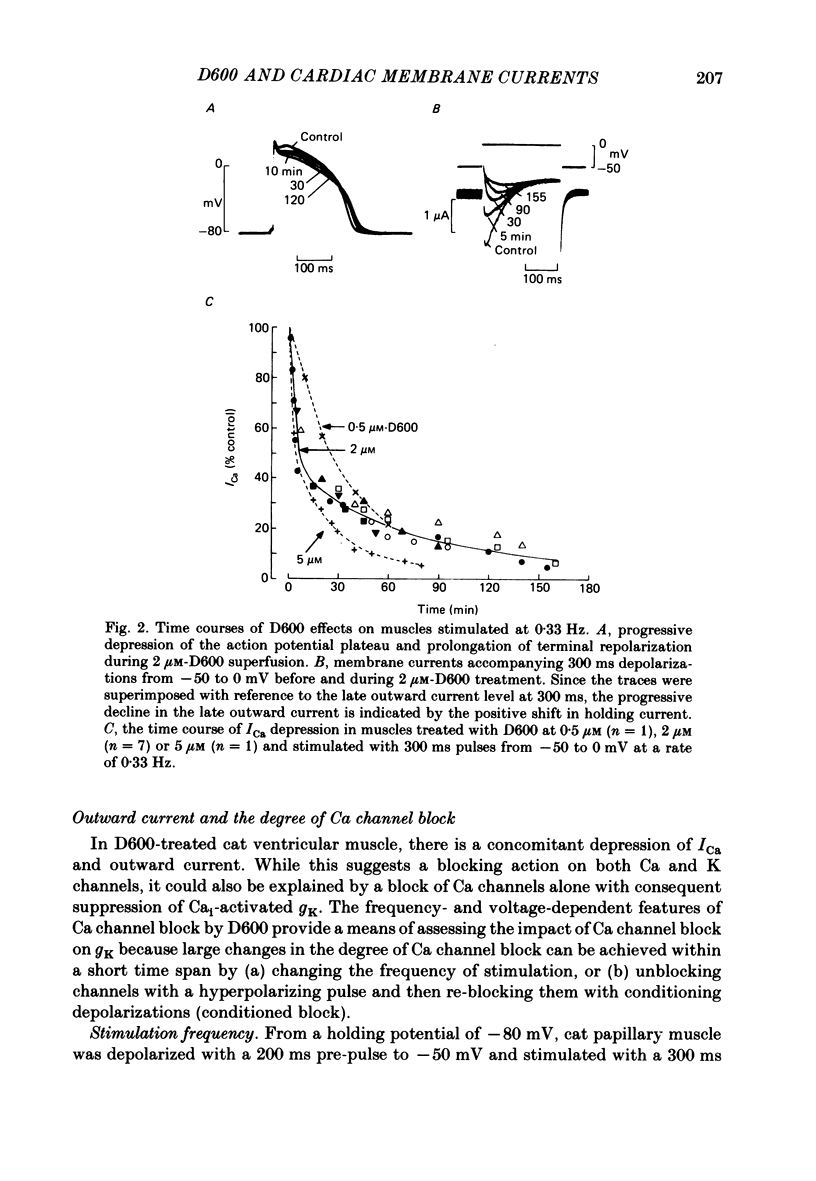
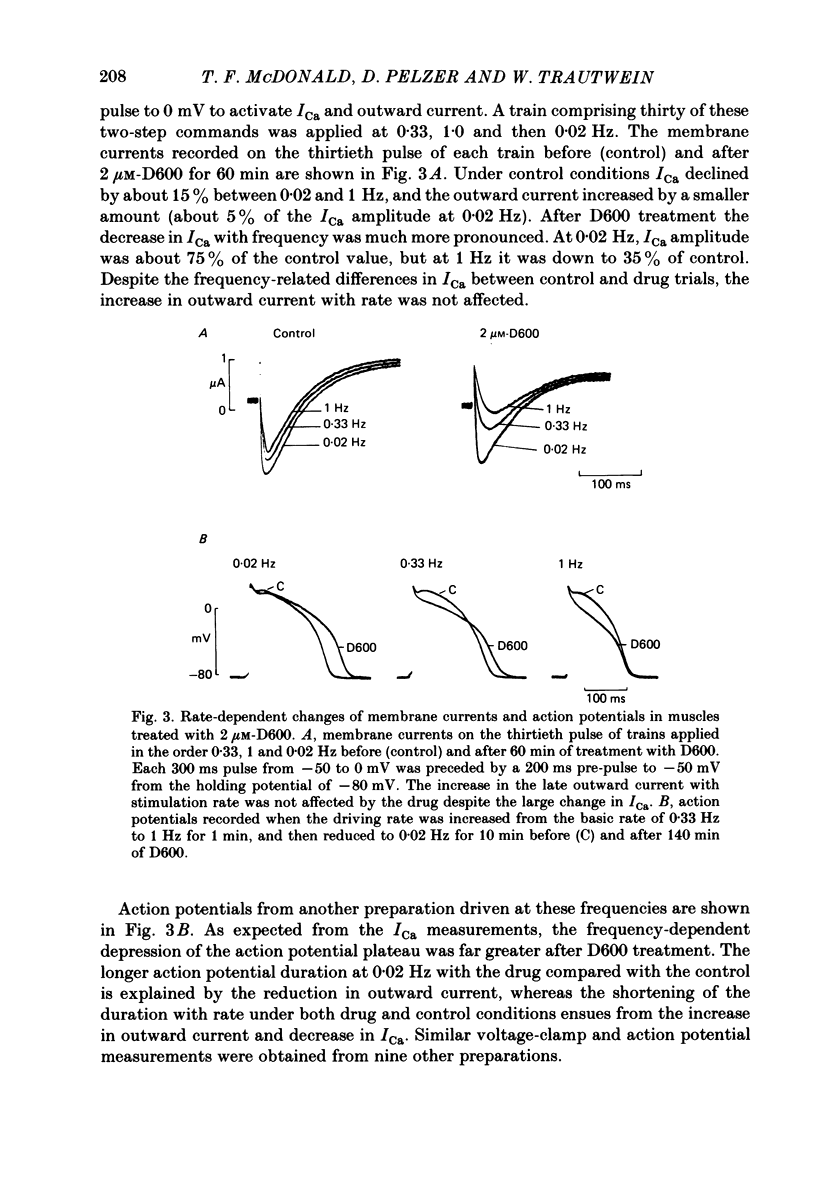
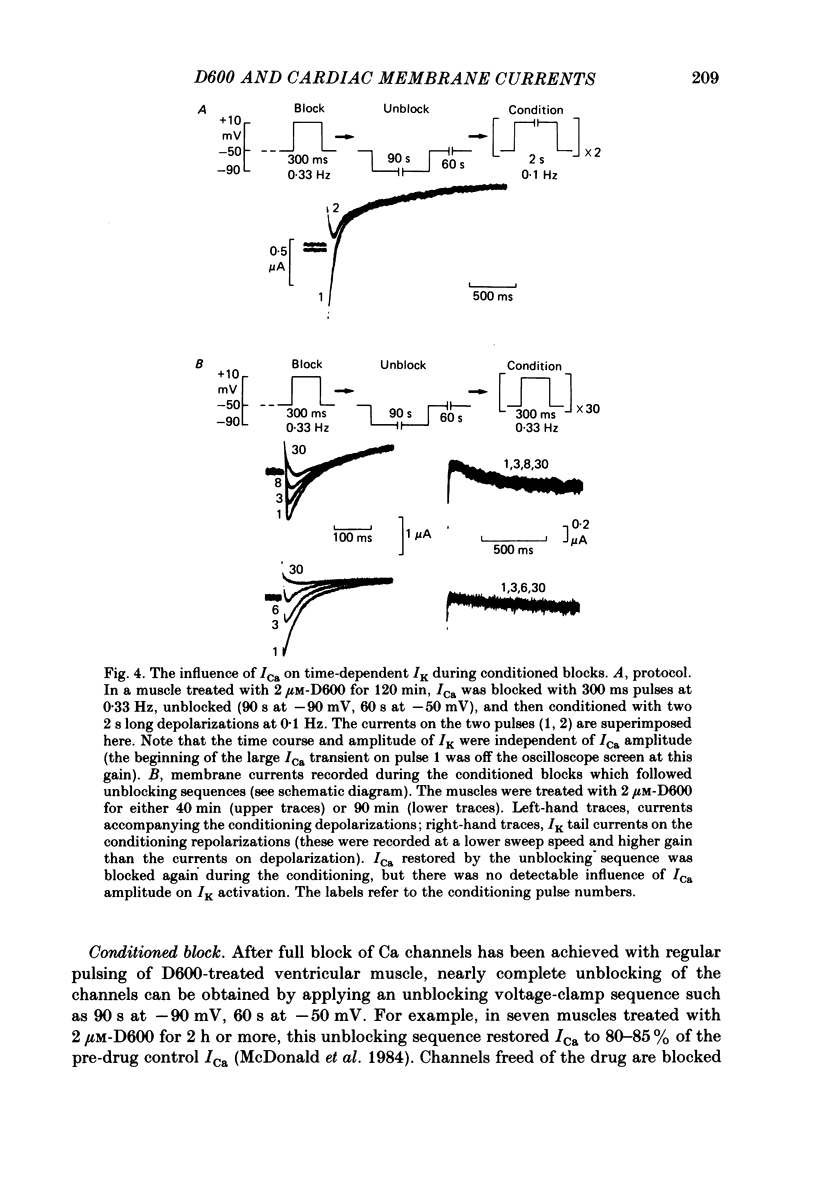
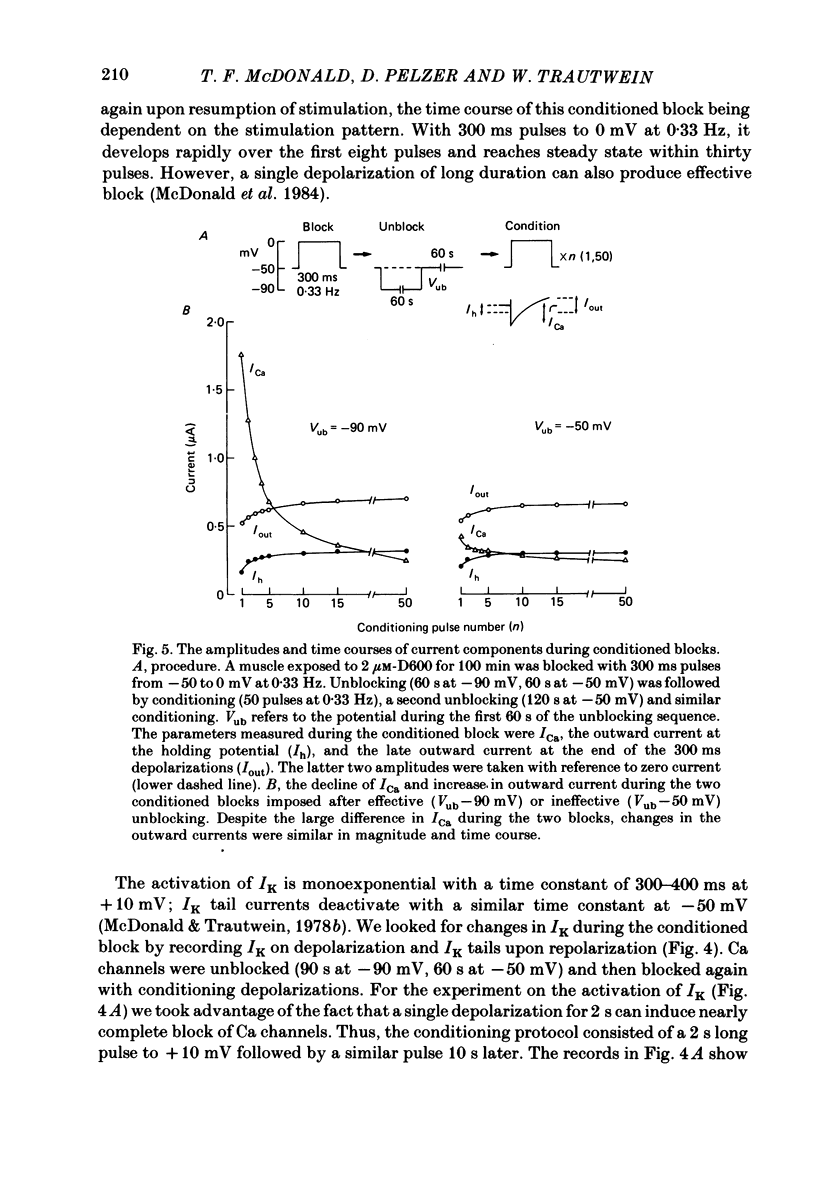
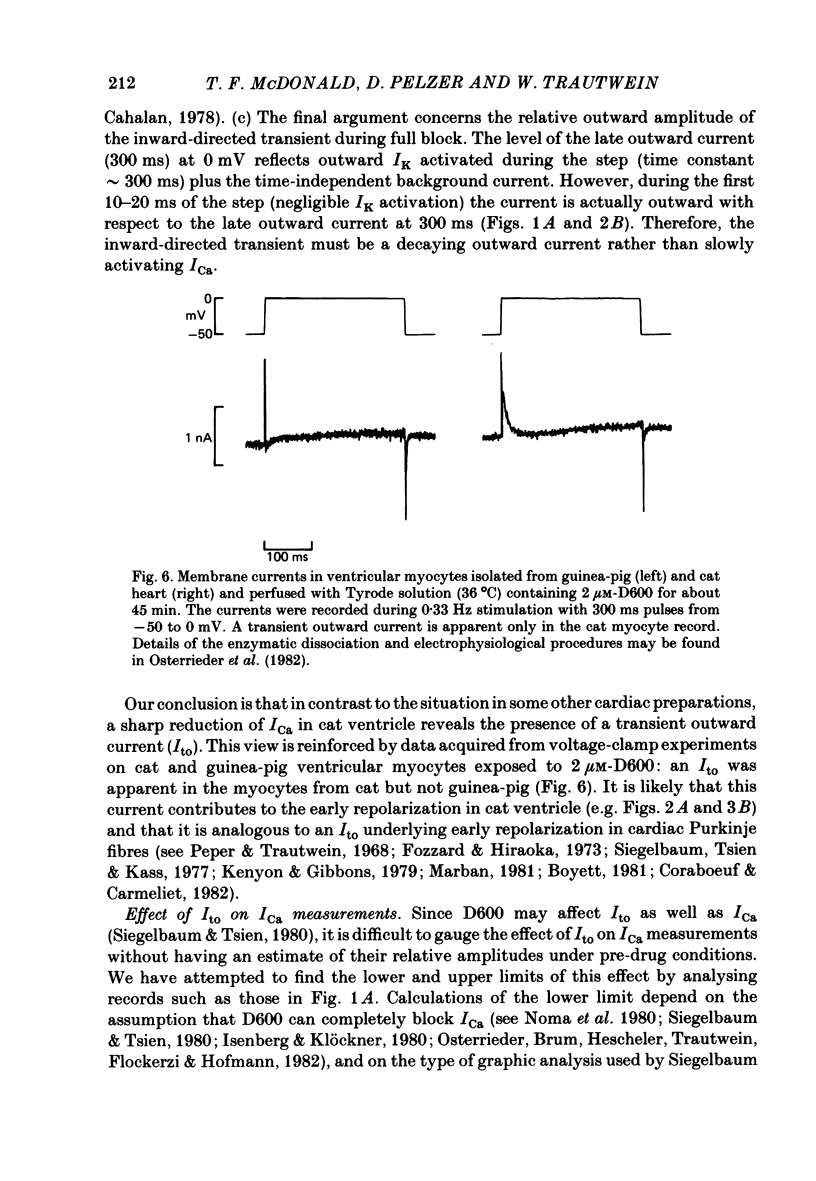
In single sucrose-gap experiments on cat ventricular muscle strands stimulated with 300 ms pulses at 0.33 Hz, 2 microM-D600 reduced the Ca-dependent slow inward current (ICa) by 50% within 5 min and more than 90% in 90-120 min. The late outward current was reduced by up to 30%. During the exposure to D600, Ca channels could be unblocked by hyperpolarizing pulses and blocked again by stimulation with depolarizing pulses. Since the degree of unblocking depended on voltage, and the degree of blocking depended on stimulation pattern, ICa amplitude could be rapidly manipulated to probe the dependence of K conductance on ICa. Under control conditions, an increase in stimulation rate from 0.02 to 1 Hz reduced ICa by 15% and increased the late outward current by a smaller amount. During exposure to D600, a similar intervention provoked a 60% reduction in ICa, but a control-like increase in the late outward current. Two other series of experiments failed to disclose a link between ICa and K conductance: when a block of Ca channels was reimposed following their unblocking, the outward currents were independent of ICa amplitude. Unblock-block experiments also provided information on the extent of steady-state ICa at 0 mV. The fraction of Ca channels not undergoing inactivation appears to be very small. During full D600 block, the inward peak of the current wave form is broad and very much delayed in comparison with pre-drug currents or currents on the first pulse following unblocking. A similar wave form was recorded in D600-treated ventricular myocytes from cat but not guinea-pig. The likely explanation is that D600 unmasks a small transient outward current in cat ventricle.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Cohen I. The voltage clamp of multicellular preparations. Prog Biophys Mol Biol. 1977;31(3):201–245. doi: 10.1016/0079-6107(78)90009-3. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassingthwaighte J. B., Fry C. H., McGuigan J. A. Relationship between internal calcium and outward current in mammalian ventricular muscle; a mechanism for the control of the action potential duration? J Physiol. 1976 Oct;262(1):15–37. doi: 10.1113/jphysiol.1976.sp011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer R., Hennekes R., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. I. Pattern of inotropic effects of the racemic compounds. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):49–68. doi: 10.1007/BF00499989. [DOI] [PubMed] [Google Scholar]
- Bayer R., Kalusche D., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. III. Effects of the optical isomers on transmembrane action potentials. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):81–97. doi: 10.1007/BF00499991. [DOI] [PubMed] [Google Scholar]
- Boyett M. R. A study of the effect of the rate of stimulation on the transient outward current in sheep cardiac Purkinje fibres. J Physiol. 1981;319:1–22. doi: 10.1113/jphysiol.1981.sp013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Courtney K. R. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J Pharmacol Exp Ther. 1975 Nov;195(2):225–236. [PubMed] [Google Scholar]
- Cranefield P. F., Aronson R. S., Wit A. L. Effect of verapamil on the noraml action potential and on a calcium-dependent slow response of canine cardiac Purkinje fibers. Circ Res. 1974 Feb;34(2):204–213. doi: 10.1161/01.res.34.2.204. [DOI] [PubMed] [Google Scholar]
- Ehara T., Daufmann R. The voltage- and time-dependent effects of (-)-verapamil on the slow inward current in isolated cat ventricular myocardium. J Pharmacol Exp Ther. 1978 Oct;207(1):49–55. [PubMed] [Google Scholar]
- Fozzard H. A., Hiraoka M. The positive dynamic current and its inactivation properties in cardiac Purkinje fibres. J Physiol. 1973 Nov;234(3):569–586. doi: 10.1113/jphysiol.1973.sp010361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Pelzer D., Trube G., Trautwein W. Does the organic calcium channel blocker D600 act from inside or outside on the cardiac cell membrane? Pflugers Arch. 1982 Jun;393(4):287–291. doi: 10.1007/BF00581411. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Kodama I., Iwamura N., Shimizu T., Toyama J., Yamada K. Effects of verapamil on canine Purkinje fibres and ventricular muscle fibres with particular reference to the alternation of action potential duration after a sudden increase in driving rate. Cardiovasc Res. 1979 Jan;13(1):1–8. doi: 10.1093/cvr/13.1.1. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Glycocalyx is not required for show inward calcium current in isolated rat heart myocytes. Nature. 1980 Mar 27;284(5754):358–360. doi: 10.1038/284358a0. [DOI] [PubMed] [Google Scholar]
- Isnberg G. Is potassium conductance of cardiac Purkinje fibres controlled by (Ca2+)? Nature. 1975 Jan 24;253(5489):273–274. doi: 10.1038/253273a0. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Siegelbaum S., Tsien R. W. Incomplete inactivation of the slow inward current in cardiac Purkinje fibres [proceedings]. J Physiol. 1976 Dec;263(1):127P–128P. [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Multiple effects of calcium antagonists on plateau currents in cardiac Purkinje fibers. J Gen Physiol. 1975 Aug;66(2):169–192. doi: 10.1085/jgp.66.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):139–157. doi: 10.1085/jgp.73.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Nishimura M., Noma A., Irisawa H. Membrane currents in the rabbit atrioventricular node cell. Pflugers Arch. 1982 Mar;393(1):15–22. doi: 10.1007/BF00582385. [DOI] [PubMed] [Google Scholar]
- Ludwig C., Nawrath H. Effects of D-600 and its optical isomers on force of contraction in cat papillary muscles and guinea-pig auricles. Br J Pharmacol. 1977 Mar;59(3):411–417. doi: 10.1111/j.1476-5381.1977.tb08394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E. Inhibition of transient outward current by intracellular ion substitution unmasks slow inward calcium current in cardiac Purkinje fibers. Pflugers Arch. 1981 Apr;390(1):102–106. doi: 10.1007/BF00582721. [DOI] [PubMed] [Google Scholar]
- Mas-Oliva J., Nayler W. G. The effect of verapamil on the Ca2+-transporting and Ca2+-ATPase activity of isolated cardiac sarcolemmal preparations. Br J Pharmacol. 1980 Dec;70(4):617–624. doi: 10.1111/j.1476-5381.1980.tb09780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCans J. L., Lindenmayer G. E., Munson R. G., Evans R. W., Schwartz A. A dissociation of positive staircase (Bowditch) from ouabain-induced positive inotropism. Circ Res. 1974 Sep;35(3):439–447. doi: 10.1161/01.res.35.3.439. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. Cat ventricular muscle treated with D600: characteristics of calcium channel block and unblock. J Physiol. 1984 Jul;352:217–241. doi: 10.1113/jphysiol.1984.sp015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. On the mechanism of slow calcium channel block in heart. Pflugers Arch. 1980 May;385(2):175–179. doi: 10.1007/BF00588699. [DOI] [PubMed] [Google Scholar]
- McDonald T. F. The slow inward calcium current in the heart. Annu Rev Physiol. 1982;44:425–434. doi: 10.1146/annurev.ph.44.030182.002233. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. Membrane currents in cat myocardium: separation of inward and outward components. J Physiol. 1978 Jan;274:193–216. doi: 10.1113/jphysiol.1978.sp012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Blocking of acetylcholine-induced channels by extracellular or intracellular application of D600. Proc R Soc Lond B Biol Sci. 1980 Dec 31;211(1182):143–150. doi: 10.1098/rspb.1980.0162. [DOI] [PubMed] [Google Scholar]
- Nathan R. D., DeHaan R. L. Voltage clamp analysis of embryonic heart cell aggregates. J Gen Physiol. 1979 Feb;73(2):175–198. doi: 10.1085/jgp.73.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath H., Eick R. E., McDonald T. F., Trautwein W. On the mechanism underlying the action of D-600 on slow inward current and tension in mammalian myocardium. Circ Res. 1977 Apr;40(4):408–414. doi: 10.1161/01.res.40.4.408. [DOI] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- Noma A., Kotake H., Irisawa H. Slow inward current and its role mediating the chronotropic effect of epinephrine in the rabbit sinoatrial node. Pflugers Arch. 1980 Oct;388(1):1–9. doi: 10.1007/BF00582621. [DOI] [PubMed] [Google Scholar]
- Noma A., Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1978 Nov 30;377(3):193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Peper K., Trautwein W. A membrane current related to the plateau of the action potential of Purkinje fibers. Pflugers Arch. 1968;303(2):108–123. doi: 10.1007/BF00592629. [DOI] [PubMed] [Google Scholar]
- Rosen M. R., Ilvento J. P., Gelband H., Merker C. Effects of verapamil on electrophysiologic properties of canine cardiac Purkinje fibers. J Pharmacol Exp Ther. 1974 May;189(2):414–422. [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W., Kass R. S. Role of intracellular calcium in the transient outward current of calf Purkinje fibres. Nature. 1977 Oct 13;269(5629):611–613. doi: 10.1038/269611a0. [DOI] [PubMed] [Google Scholar]
- Siegl P. K., McNeill J. H. The negative inotropic potency of compound D 600 in rat, guinea pig, and rabbit cardiac preparations. Can J Physiol Pharmacol. 1980 Dec;58(12):1406–1411. doi: 10.1139/y80-213. [DOI] [PubMed] [Google Scholar]
- Singh B. N. A fourth class of anti-dysrhythmic action? Effect of verapamil on ouabain toxicity, on atrial and ventricular intracellular potentials, and on other features of cardiac function. Cardiovasc Res. 1972 Mar;6(2):109–119. doi: 10.1093/cvr/6.2.109. [DOI] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit A. L., Cranefield P. F. Effect of verapamil on the sinoatrial and atrioventricular nodes of the rabbit and the mechanism by which it arrests reentrant atrioventricular nodal tachycardia. Circ Res. 1974 Sep;35(3):413–425. doi: 10.1161/01.res.35.3.413. [DOI] [PubMed] [Google Scholar]


