Abstract
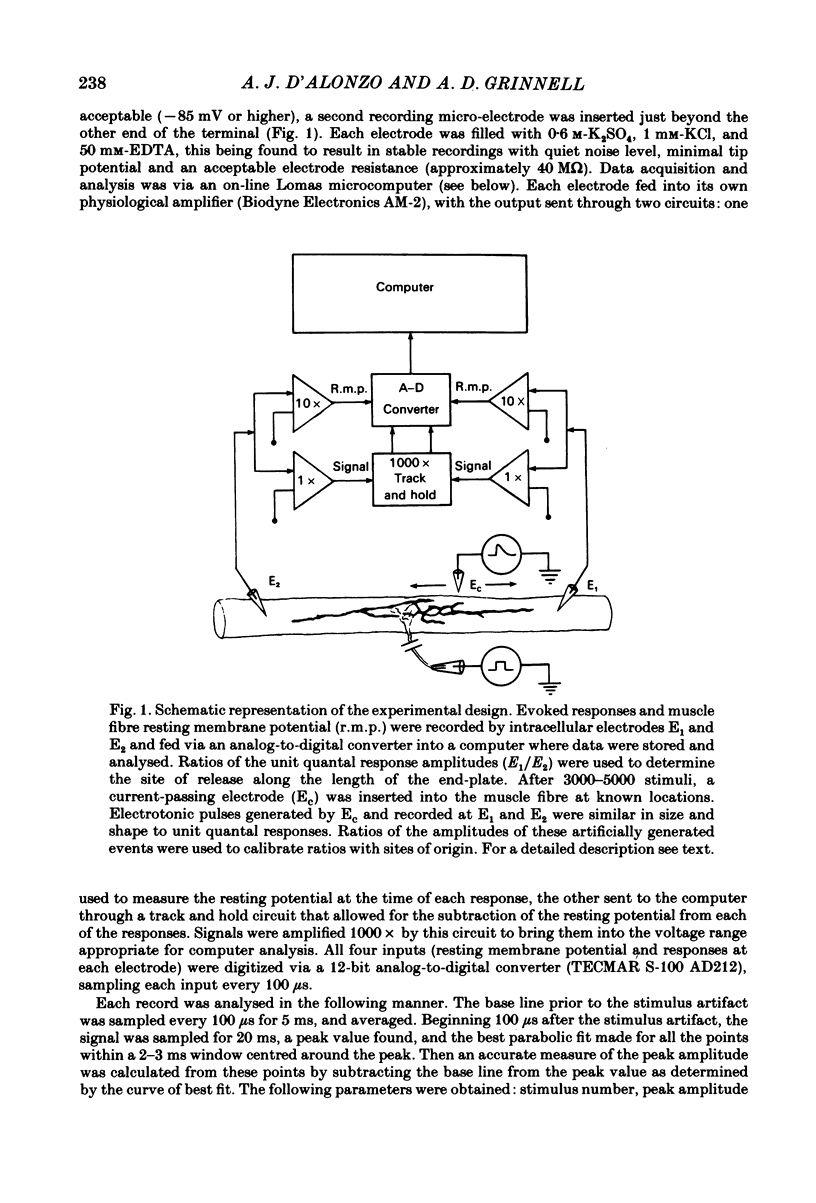
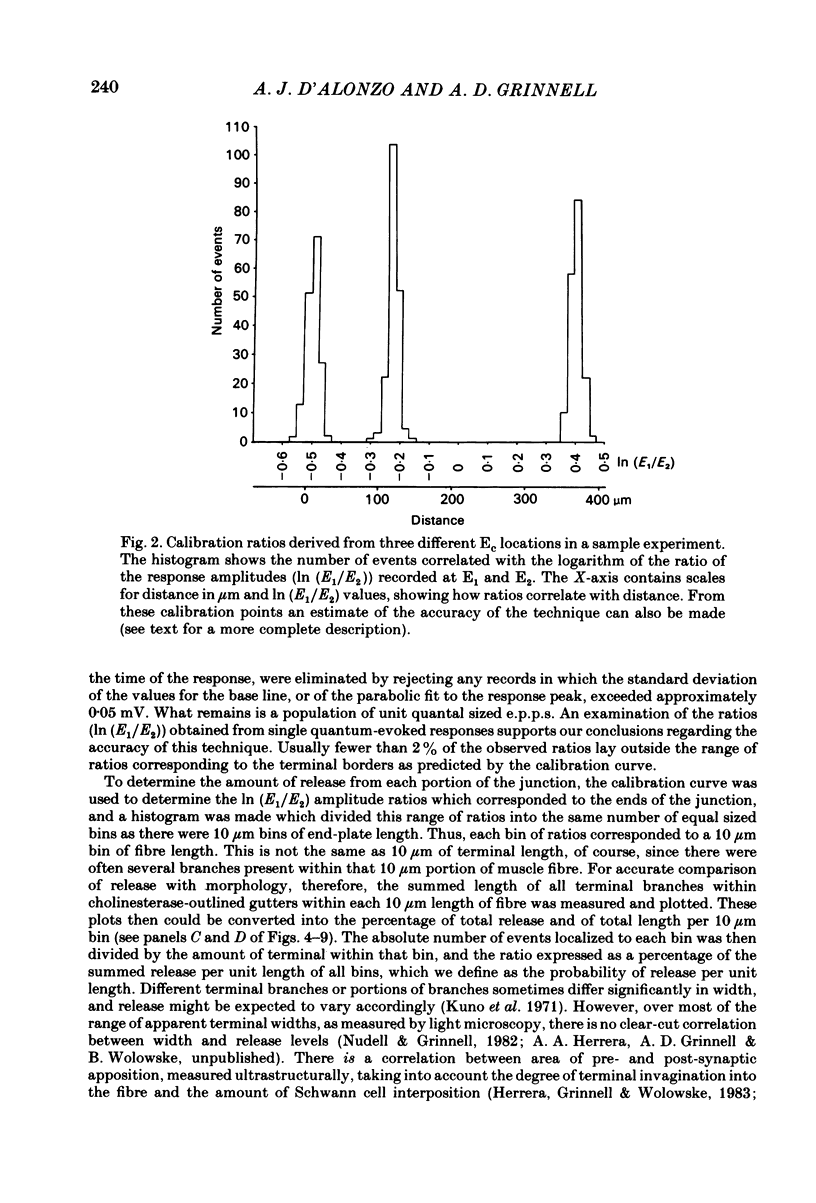
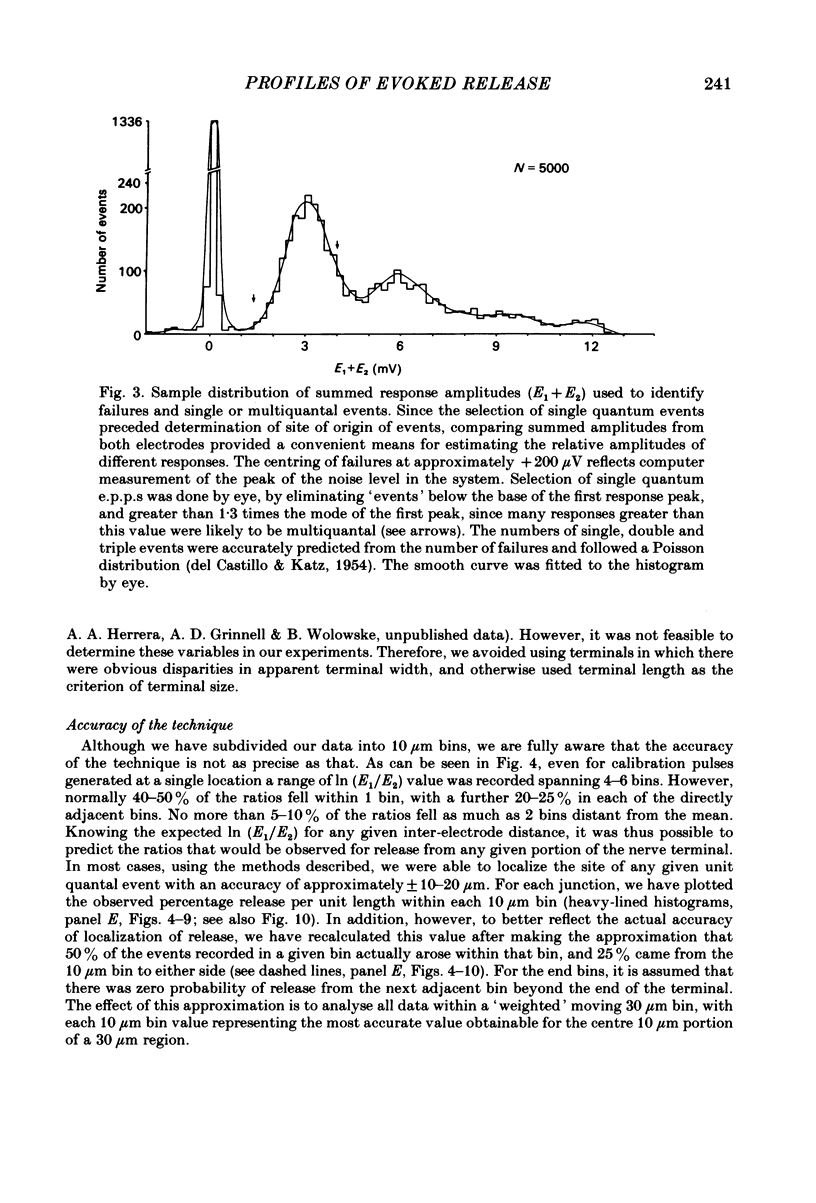
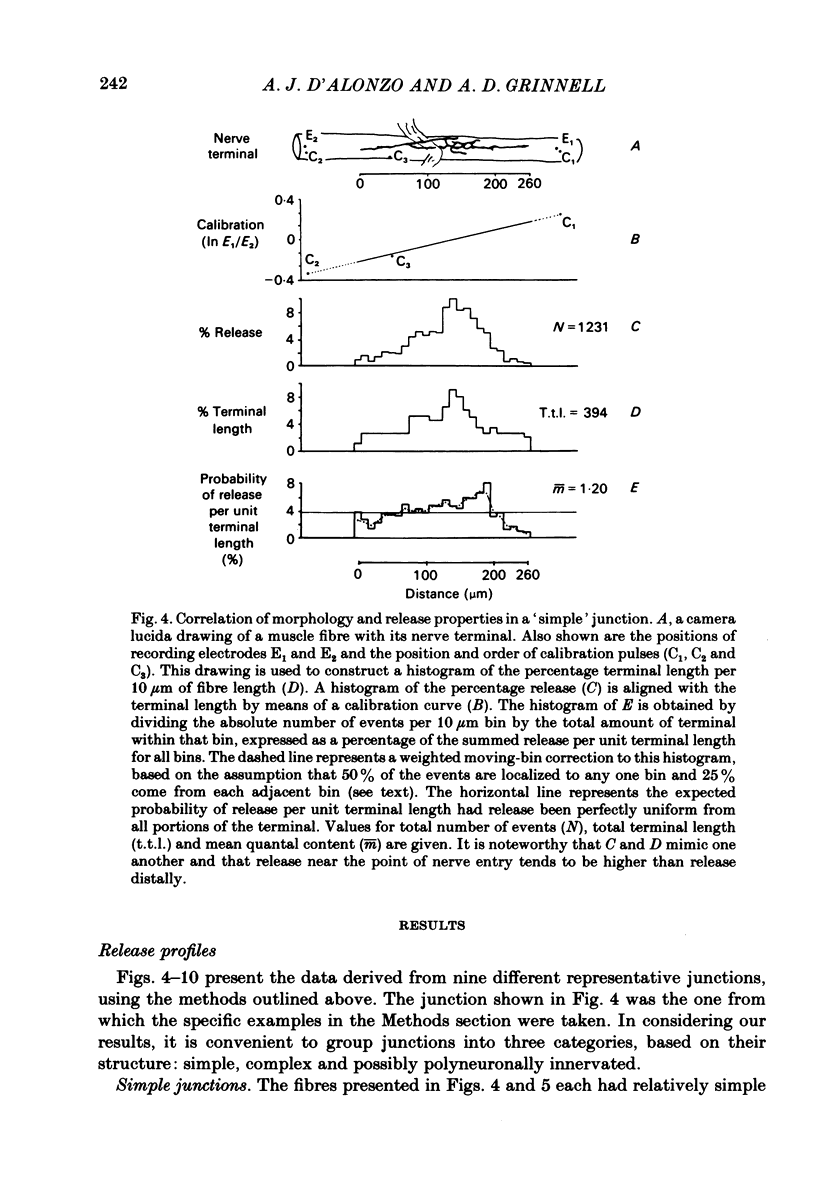
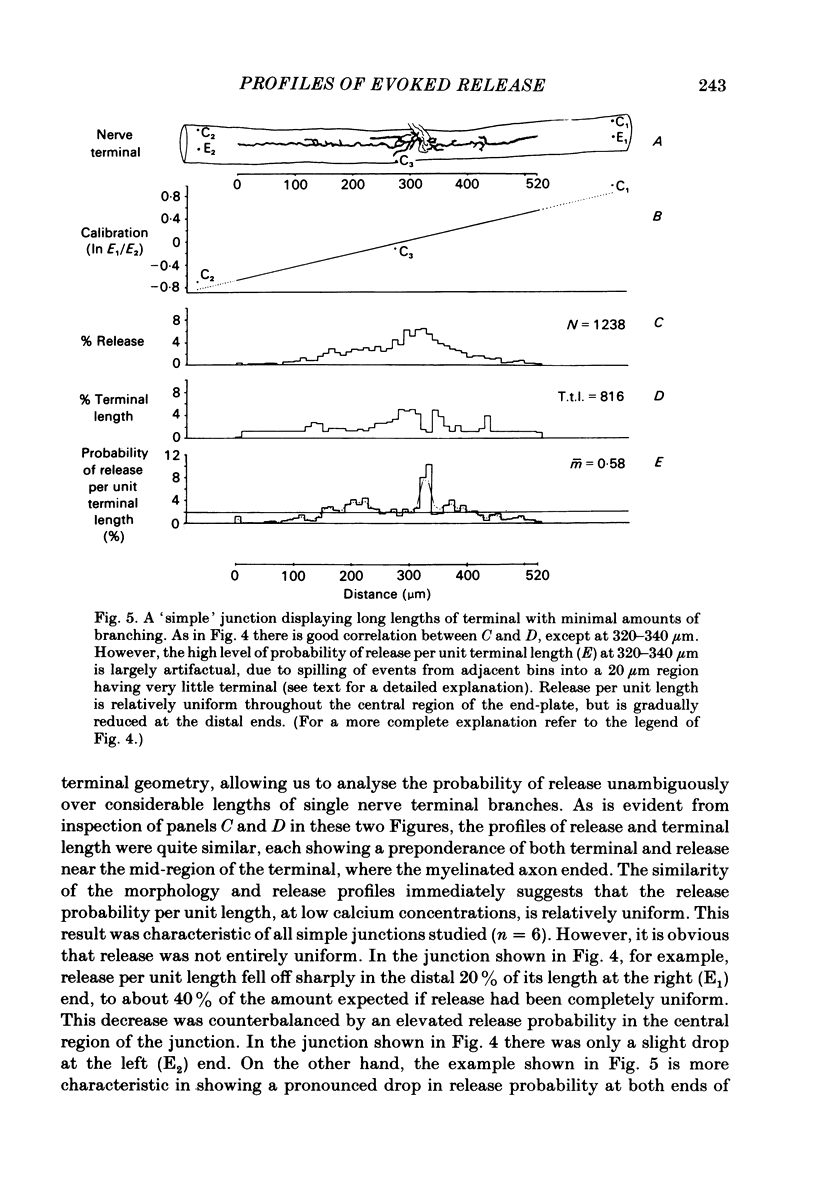
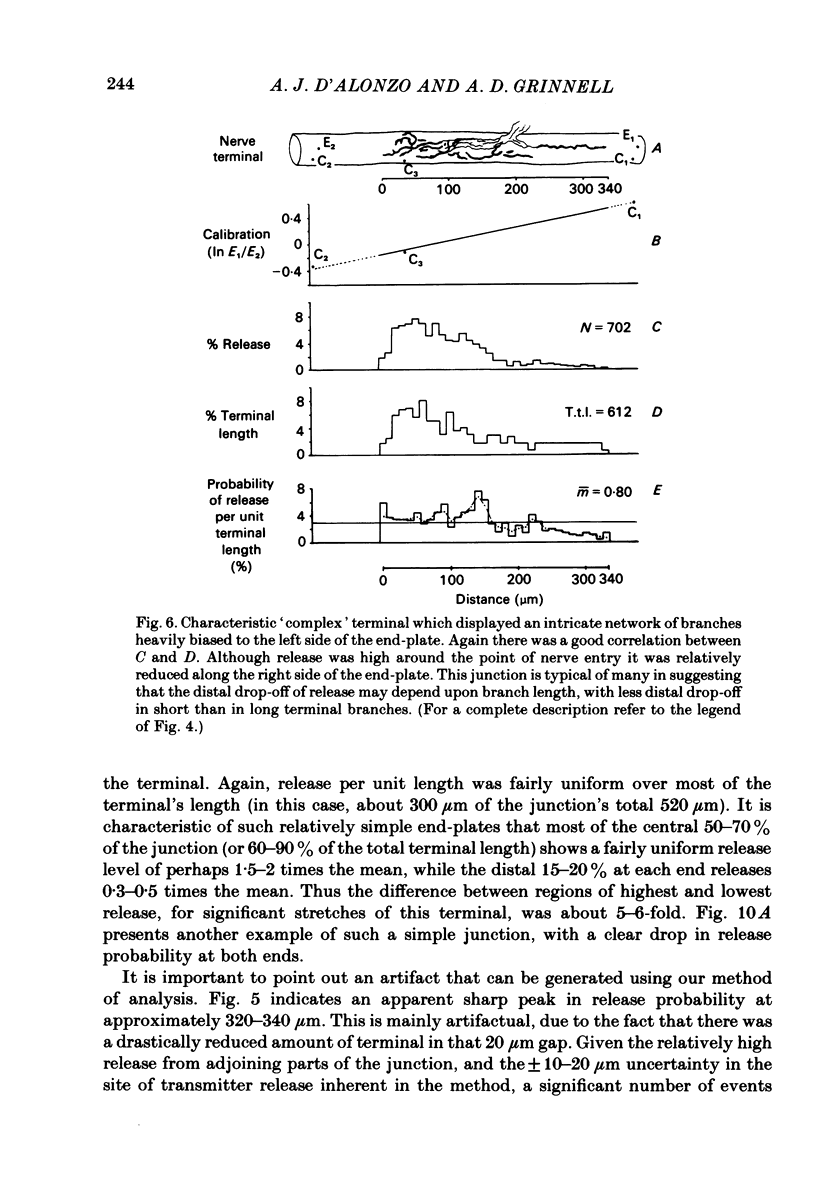
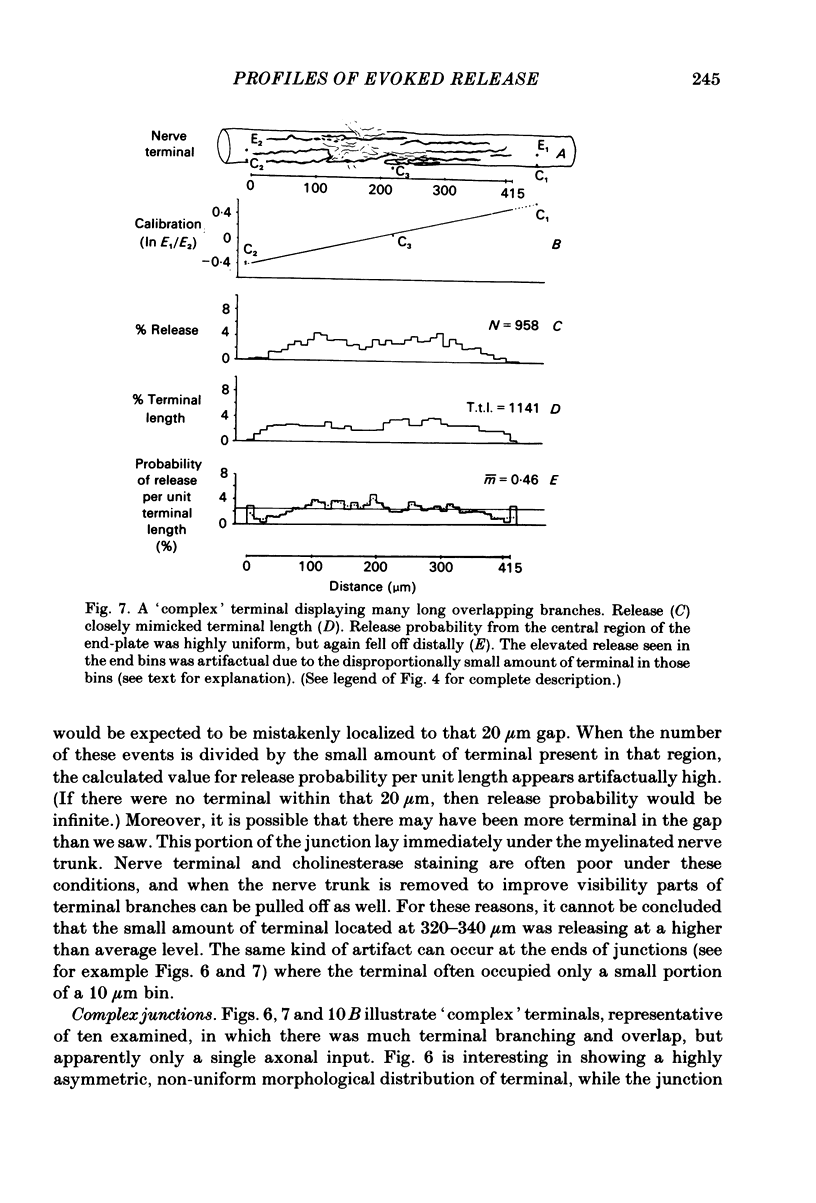
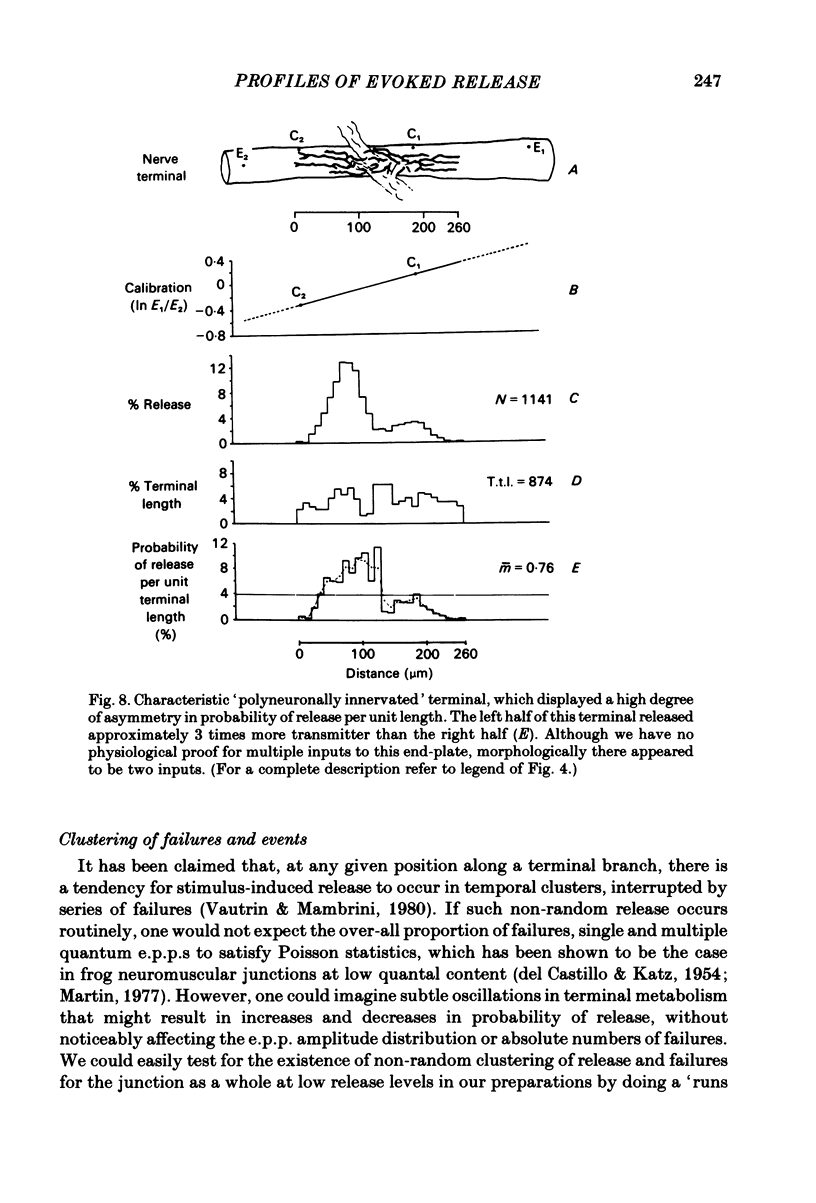
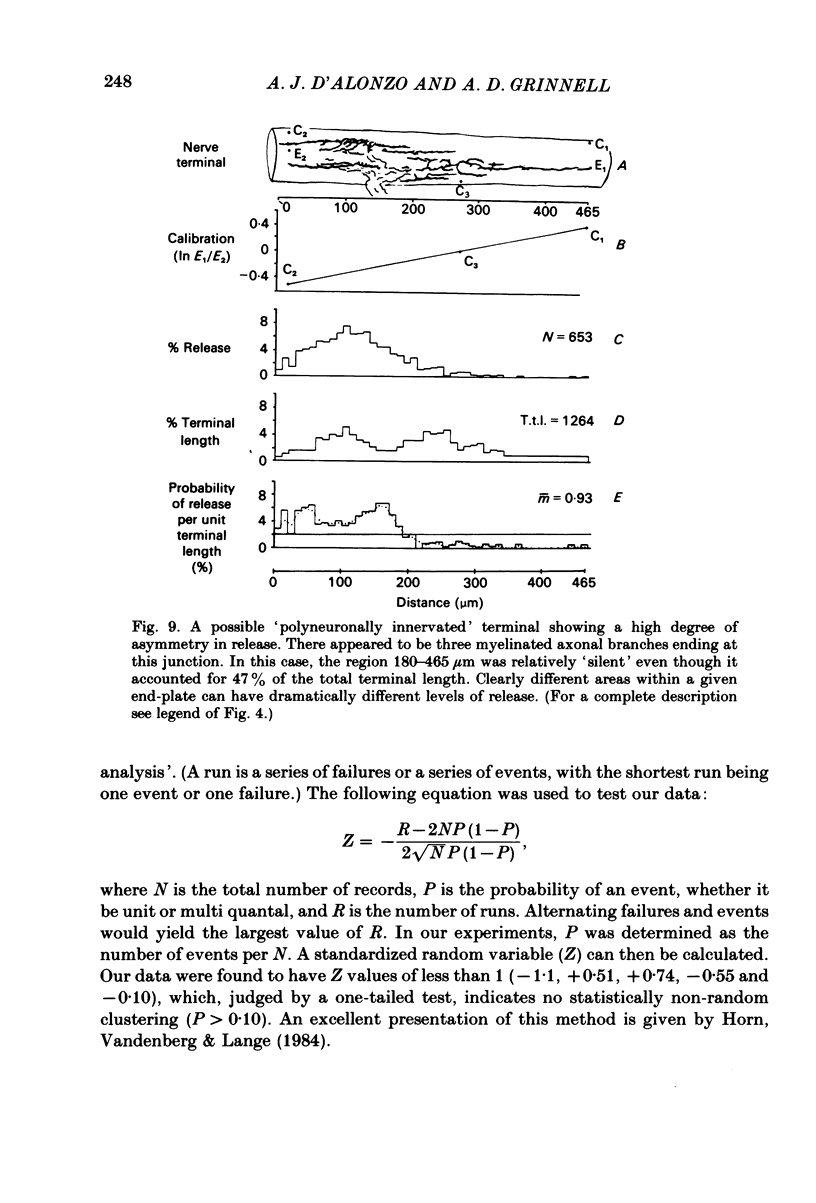
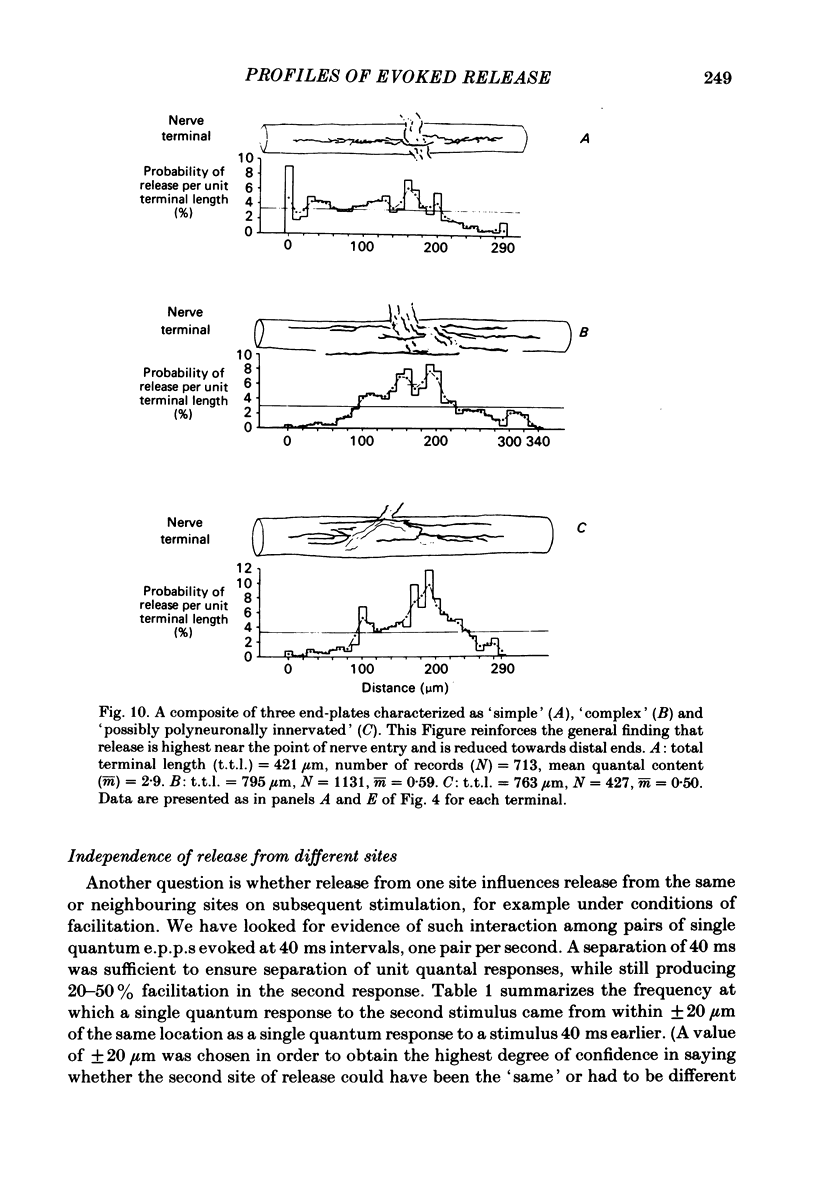
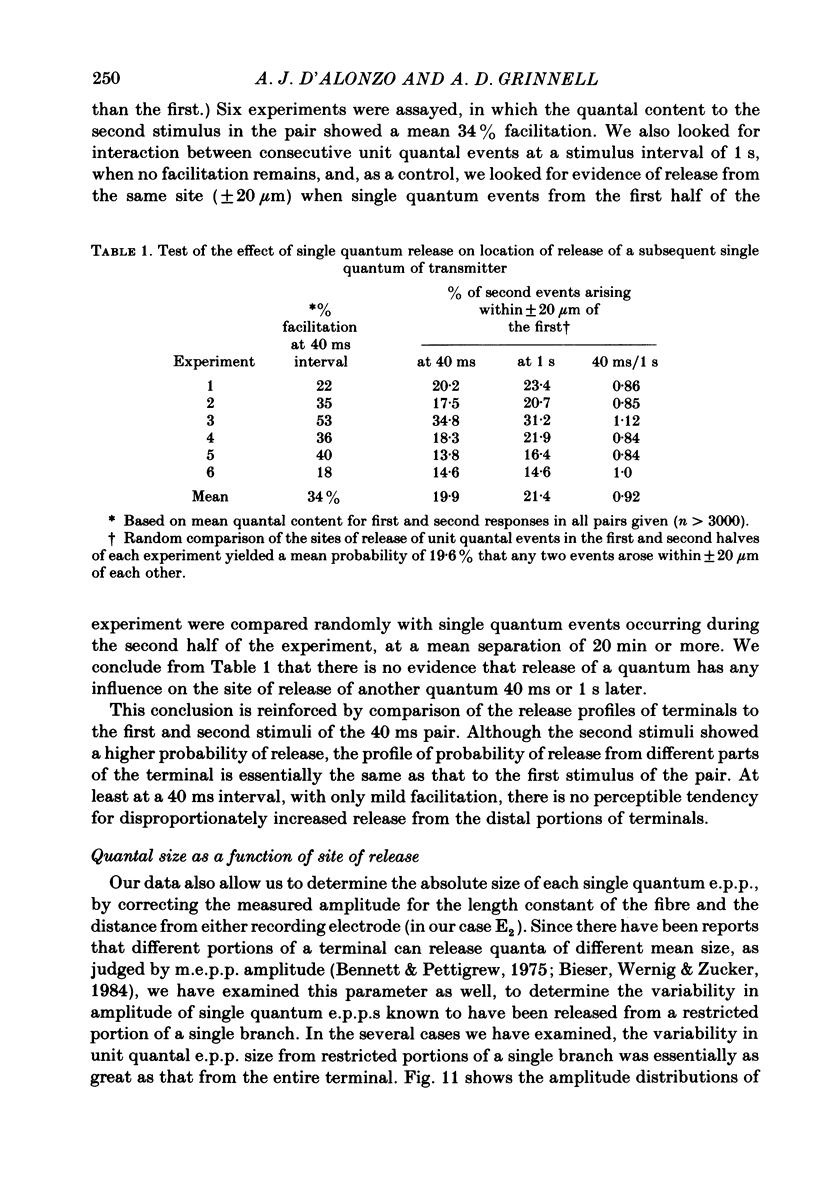
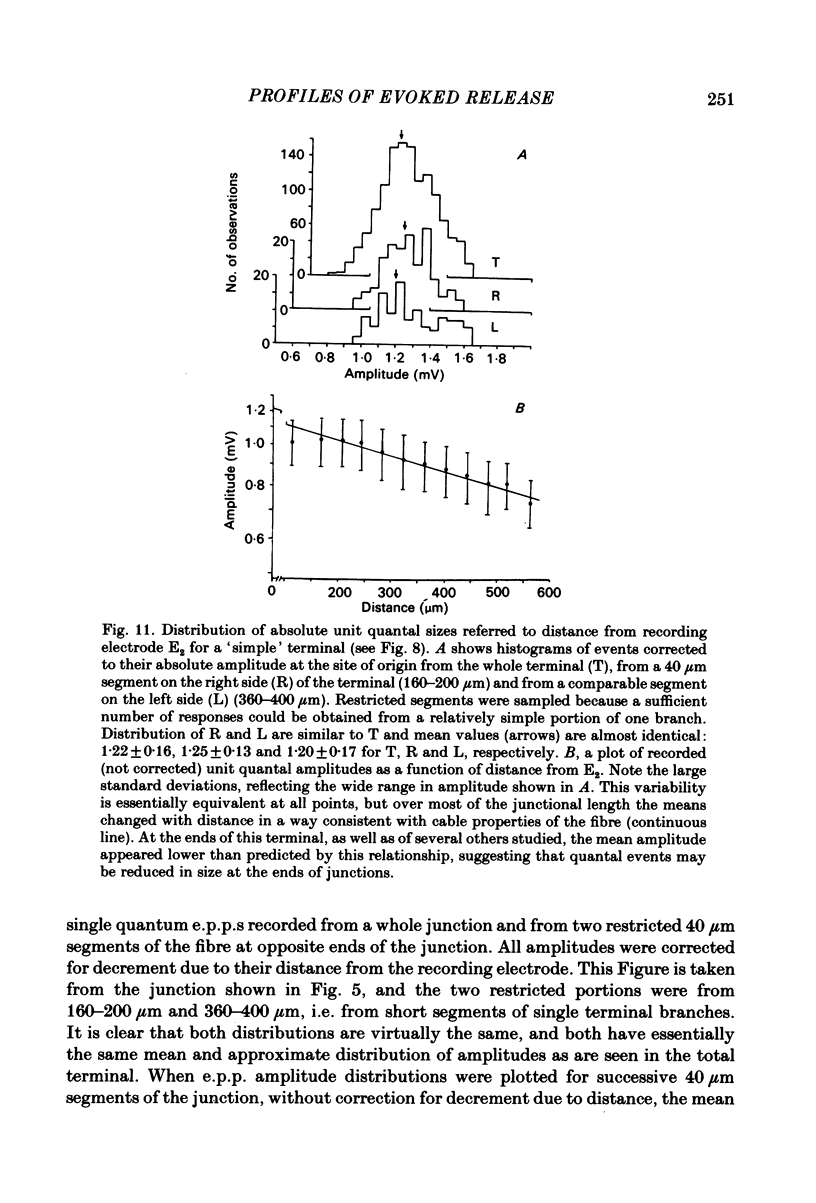
In order to determine the relative probability of evoked transmitter release from different parts of frog motor nerve terminals, a technique has been developed in which single quantum end-plate potentials (e.p.p.s) are recorded by two intracellular electrodes, located at opposite ends of identified junctions. The log of the ratio of the amplitudes recorded simultaneously at the two electrodes is a linear function of the distance of the site of origin of the event from each of the two electrodes. Using online computer data acquisition and analysis, and current pulses at known locations for spatial calibration, it is possible to localize the site of single quantum e.p.p.s to within +/- 10-20 micron. Using the frog cutaneous pectoris neuromuscular preparation and a low calcium, high magnesium Ringer solution to ensure mostly single quantum events and failures, several thousand responses were recorded from each junction, allowing construction of a profile of the numbers of single quantum events arising from each portion of the junction. By comparison of junctional morphology and release profiles, it is possible to construct a probability of release per unit length profile for the entire junction. This technique has several advantages over localization of release events by measurements of extracellular synaptic currents. It was found that, for most junctions, the central 60-90% of the terminal exhibited relatively uniform probability of release, with highest levels typically near the point where the axon first contacted the muscle fibre, or in regions with many short terminal branches. However, no instances have been found in which a small region of terminal (10% or less) showed extraordinarily high release levels (30-50% of the total release from the junction). Characteristically, but not invariably, there is reduced release near the ends of terminal branches, especially the longer branches, where release per unit length could be as little as 5-10% of that in proximal portions. Some junctions had large regions of terminal that released very little transmitter. These also showed multiple myelineated axonal inputs, and may have been polyneuronally innervated junctions in which one of the inputs was much weaker than the other.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzil A. P., Bieser A., Wernig A. Light and electron microscopic identification of nerve terminal sprouting and retraction in normal adult frog muscle. J Physiol. 1984 May;350:393–399. doi: 10.1113/jphysiol.1984.sp015207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. Quantal independence and uniformity of presynaptic release kinetics at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):665–689. doi: 10.1113/jphysiol.1972.sp010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet M. R., Lavidis N. A. Variation in quantal secretion at different release sites along developing and mature motor terminal branches. Brain Res. 1982 Sep;281(1):1–9. doi: 10.1016/0165-3806(82)90107-9. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. The effect of calcium ions on the secretion of quanta evoked by an impulse at nerve terminal release sites. J Gen Physiol. 1979 Oct;74(4):429–456. doi: 10.1085/jgp.74.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieser A., Wernig A., Zucker H. Different quantal responses within single frog neuromuscular junctions. J Physiol. 1984 May;350:401–412. doi: 10.1113/jphysiol.1984.sp015208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M., Schmidt R. F. Potential changes recorded from the frog motor nerve terminal during its activation. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(1):56–80. doi: 10.1007/BF00362454. [DOI] [PubMed] [Google Scholar]
- Couteaux R., Pécot-Dechavassine M. Vésicules synaptiques et poches au niveau des "zones actives" de la jonction neuromusculaire. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2346–2349. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey D. F., Bennett M. R. Variation in the size of synaptic contacts along developing and mature motor terminal branches. Brain Res. 1982 Sep;281(1):11–22. doi: 10.1016/0165-3806(82)90108-0. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D., Herrera A. A. Physiological regulation of synaptic effectiveness at frog neuromuscular junctions. J Physiol. 1980 Oct;307:301–317. doi: 10.1113/jphysiol.1980.sp013436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Katz B., Miledi R. The reduction of endplate responses by botulinum toxin. Proc R Soc Lond B Biol Sci. 1981 Nov 24;213(1193):489–493. doi: 10.1098/rspb.1981.0077. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Ribchester R. R. The relationship between end-plate size and transmitter release in normal and dystrophic muscles of the mouse. J Physiol. 1979 Nov;296:245–265. doi: 10.1113/jphysiol.1979.sp013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A. A., Grinnell A. D. Contralateral denervation causes enhanced transmitter release from frog motor nerve terminals. Nature. 1981 Jun 11;291(5815):495–497. doi: 10.1038/291495a0. [DOI] [PubMed] [Google Scholar]
- Herrera A. A., Grinnell A. D. Transmitter release from frog motor nerve terminals depends on motor unit size. Nature. 1980 Oct 16;287(5783):649–651. doi: 10.1038/287649a0. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A., Lange K. Statistical analysis of single sodium channels. Effects of N-bromoacetamide. Biophys J. 1984 Jan;45(1):323–335. doi: 10.1016/S0006-3495(84)84158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J. THE LOCALIZATION OF CHOLINESTERASE ACTIVITY IN RAT CARDIAC MUSCLE BY ELECTRON MICROSCOPY. J Cell Biol. 1964 Nov;23:217–232. doi: 10.1083/jcb.23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of local blockage of motor nerve terminals. J Physiol. 1968 Dec;199(3):729–741. doi: 10.1113/jphysiol.1968.sp008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C. P. Electrophysiological and freeze-fracture studies of changes following denervation at frog neuromuscular junctions. J Physiol. 1981 Dec;321:627–639. doi: 10.1113/jphysiol.1981.sp014007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinsky M. S., Decino P. A. Histological staining of pre- and postsynaptic components of amphibian neuromuscular junctions. J Neurocytol. 1980 Jun;9(3):305–320. doi: 10.1007/BF01181539. [DOI] [PubMed] [Google Scholar]
- Mallart A. Presynaptic currents in frog motor endings. Pflugers Arch. 1984 Jan;400(1):8–13. doi: 10.1007/BF00670529. [DOI] [PubMed] [Google Scholar]
- Nudell B. M., Grinnell A. D. Inverse relationship between transmitter release and terminal length in synapses on frog muscle fibers of uniform input resistance. J Neurosci. 1982 Feb;2(2):216–224. doi: 10.1523/JNEUROSCI.02-02-00216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudell B. M., Grinnell A. D. Regulation of synaptic position, size, and strength in anuran skeletal muscle. J Neurosci. 1983 Jan;3(1):161–176. doi: 10.1523/JNEUROSCI.03-01-00161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper K., Dreyer F., Sandri C., Akert K., Moor H. Structure and ultrastructure of the frog motor endplate. A freeze-etching study. Cell Tissue Res. 1974 Jun 24;149(4):437–455. doi: 10.1007/BF00223024. [DOI] [PubMed] [Google Scholar]
- Pockett S., Slack J. R. Pruning of axonal trees results in increased efficacy of surviving nerve terminals. Brain Res. 1982 Jul 15;243(2):350–353. doi: 10.1016/0006-8993(82)90259-1. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A. Localization of active sites in the neuromuscular junction of the frog. Brain Res. 1976 Dec 10;118(1):63–72. doi: 10.1016/0006-8993(76)90841-6. [DOI] [PubMed] [Google Scholar]
- Wernig A., Pécot-Dechavassine M., Stover H. Sprouting and regression of the nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare. J Neurocytol. 1980 Jun;9(3):278–303. doi: 10.1007/BF01181538. [DOI] [PubMed] [Google Scholar]


