Abstract
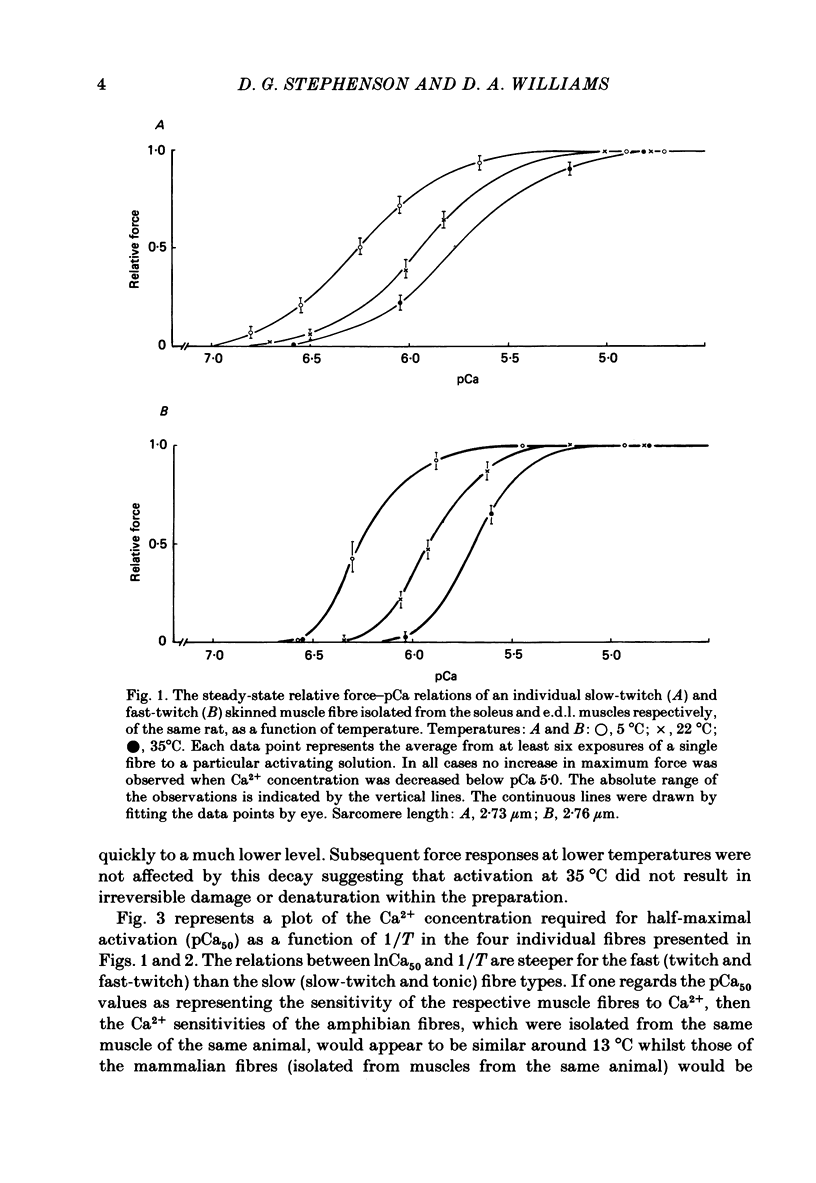
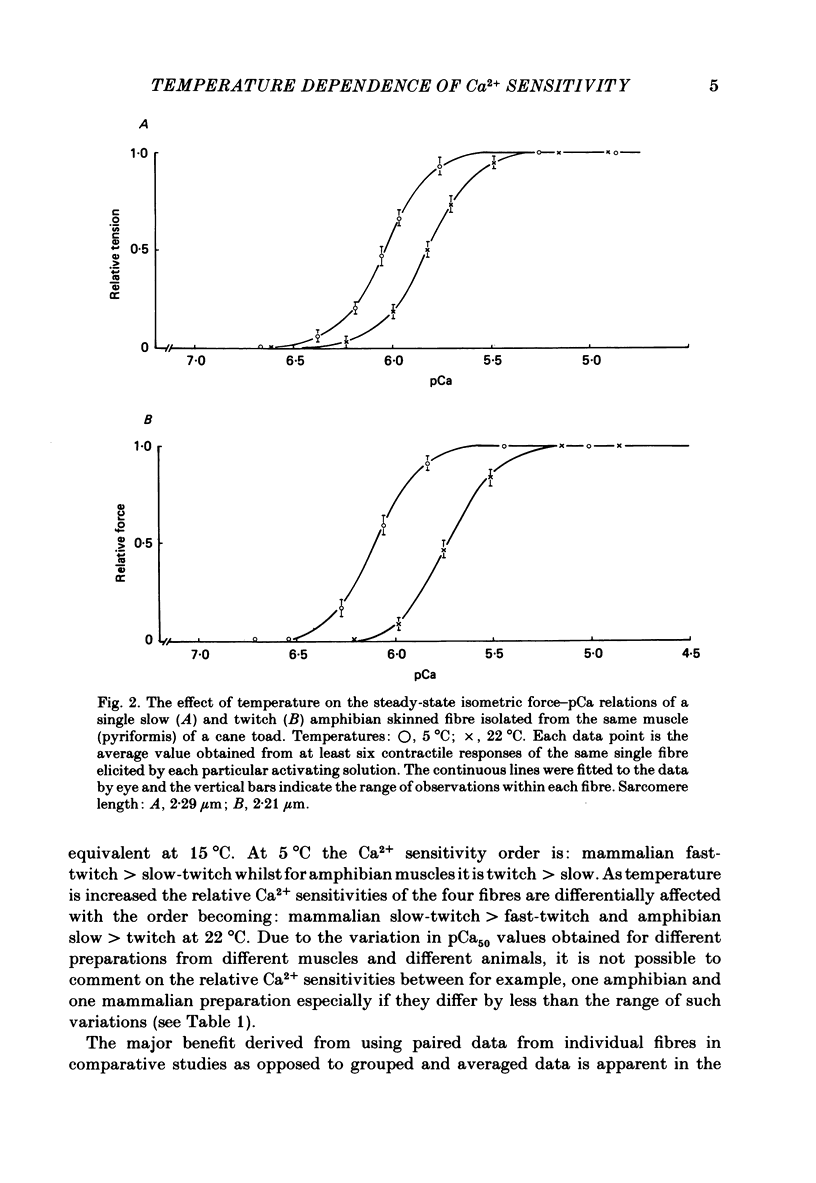
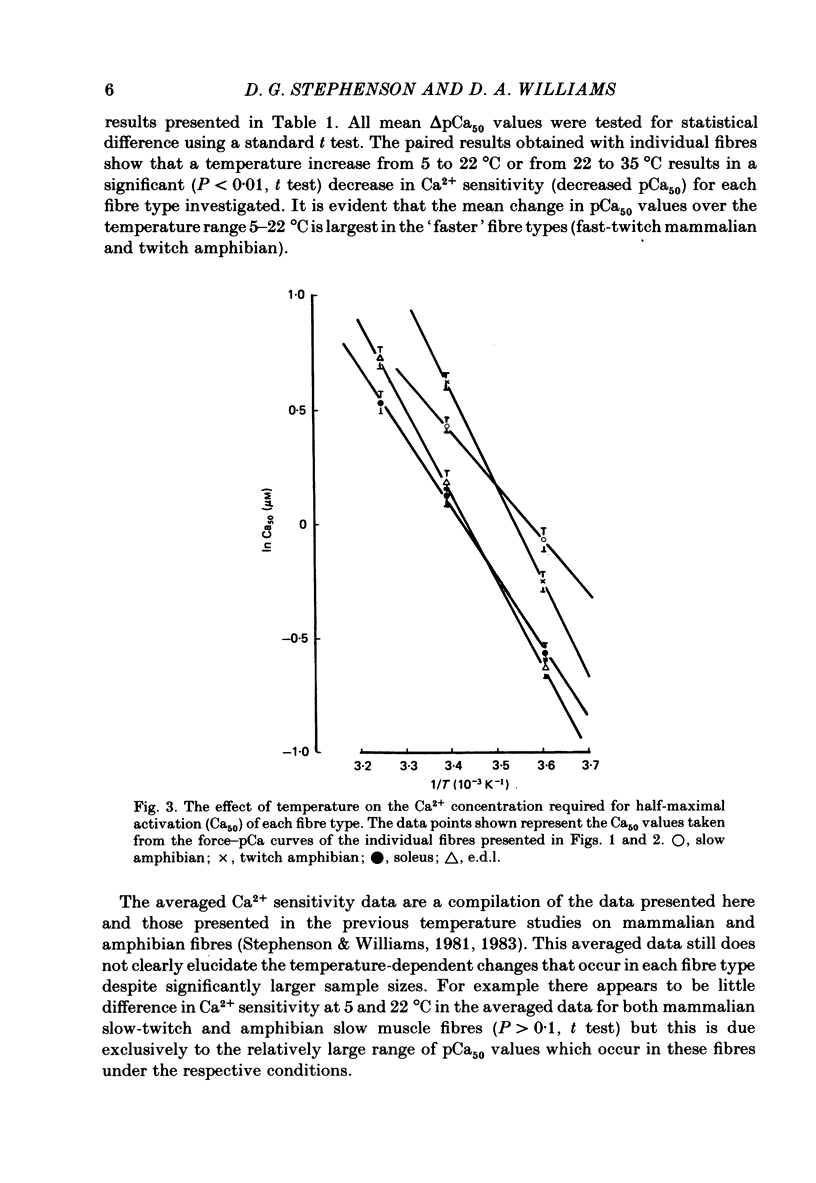
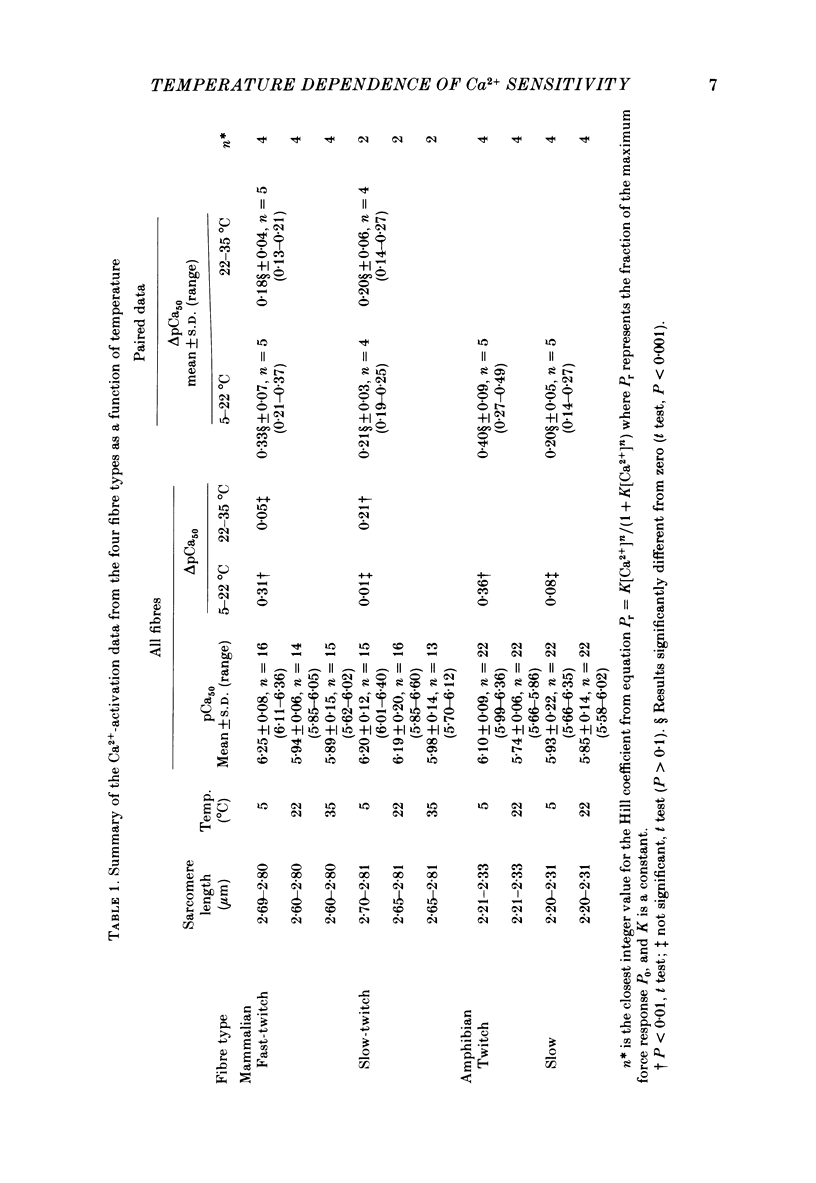
Single mechanically skinned muscle fibres of different types (fast- and slow-twitch mammalian; slow and twitch amphibian) were successively activated in solutions of various Ca2+ concentrations at different temperatures. An increase in temperature from 5 to 22 degrees C reversibly shifted the isometric steady-state force-pCa curves towards higher Ca2+ concentration for individual fibres of each of the muscle types. A further increase in temperature to 35 degrees C in mammalian fibres resulted in an additional decrease in Ca2+ sensitivity. The temperature dependence of Ca2+ sensitivity was greater in the 'faster' fibre types: fast-twitch greater than slow-twitch; twitch greater than slow. The maximum isometric force response, P0, of both rat and toad skinned fibres was found to be strongly dependent on temperature below 22 degrees C. No detectable force could be induced by Ca2+ in mammalian muscle fibres at 0-1 degree C while in toad fibres P0 decreased by about 90% when temperature dropped from 20 to 0 degree C. Since in mechanically skinned fibres of other amphibians (Bufo bufo, Rana species) P0 is only marginally affected it is likely that the P0-temperature relations are indicative of the range of temperature over which the muscles are normally functional. The P0-temperature relations of skinned muscle fibres closely resembled the P0-temperature relations of tetanically stimulated intact muscle preparations from the same species of animals suggesting that the contractile apparatus is mainly responsible for the variation of force response with temperature in intact muscle.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Moisescu D. G. Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J Physiol. 1977 Sep;270(3):627–652. doi: 10.1113/jphysiol.1977.sp011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
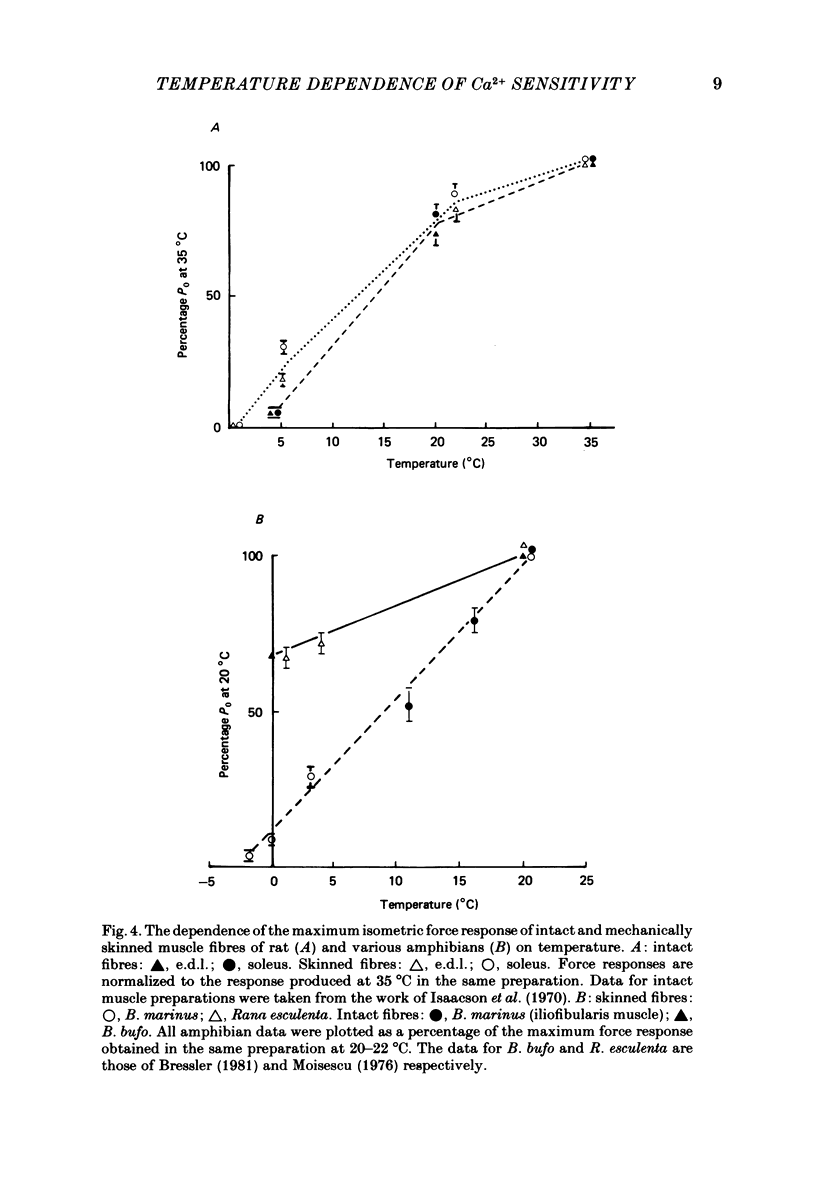
- Bressler B. H. Isometric contractile properties and instantaneous stiffness of amphibian skeletal muscle in the temperature range from 0 to 20 degrees C. Can J Physiol Pharmacol. 1981 Jun;59(6):548–554. doi: 10.1139/y81-082. [DOI] [PubMed] [Google Scholar]
- CULLINGHAM P. J., LIND A. R., MORTON R. J. The maximal isometric tetanic tensions developed by mammalian muscle, in situ, at different temperatures. Q J Exp Physiol Cogn Med Sci. 1960 Apr;45:142–156. doi: 10.1113/expphysiol.1960.sp001452. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Close R., Hoh J. F. Influence of temperature on isometric contractions of rat skeletal muscles. Nature. 1968 Mar 23;217(5134):1179–1180. doi: 10.1038/2171179a0. [DOI] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J., Tice L. W. Calcium activation of frog slow muscle fibres. J Physiol. 1967 Jan;188(2):261–271. doi: 10.1113/jphysiol.1967.sp008137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. Vertebrate slow muscle fibers. Physiol Rev. 1970 Jan;50(1):40–62. doi: 10.1152/physrev.1970.50.1.40. [DOI] [PubMed] [Google Scholar]
- Hill D. K. Resting tension and the form of the twitch of rat skeletal muscle at low temperature. J Physiol. 1972 Feb;221(1):161–171. doi: 10.1113/jphysiol.1972.sp009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson A., Hinkes M. J., Taylor S. R. Contracture and twitch potentiation of fast and slow muscles of the rat at 20 and 37 C. Am J Physiol. 1970 Jan;218(1):33–41. doi: 10.1152/ajplegacy.1970.218.1.33. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L. Effects of calcium and ionic strength on shortening velocity and tension development in frog skinned muscle fibres. J Physiol. 1981 Feb;311:179–199. doi: 10.1113/jphysiol.1981.sp013580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. The force-velocity relation of isolated twitch and slow muscle fibres of Xenopus laevis. J Physiol. 1978 Oct;283:501–521. doi: 10.1113/jphysiol.1978.sp012516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G., Thieleczek R. Sarcomere length effects on the Sr2+- and Ca2+-activation curves in skinned frog muscle fibres. Biochim Biophys Acta. 1979 Apr 11;546(1):64–76. doi: 10.1016/0005-2728(79)90170-1. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Onoda Y. Barium and strontium can substitute for calcium in noradrenaline output induced by excess potassium in the guinea-pig. J Physiol. 1980 Aug;305:59–71. doi: 10.1113/jphysiol.1980.sp013349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. D., Hsu F. J., Pownall H. J. Thermodynamics of Ca2+ binding to troponin-C. J Biol Chem. 1977 Apr 10;252(7):2452–2454. [PubMed] [Google Scholar]
- Ranatunga K. W., Wylie S. R. Temperature-dependent transitions in isometric contractions of rat muscle. J Physiol. 1983 Jun;339:87–95. doi: 10.1113/jphysiol.1983.sp014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secrist D. J., Kerrick W. G. Associated changes in Ca2+ and Sr2+ activation properties and fiber proteins in cross-reinnervated rabbit soleus. Pflugers Arch. 1980 Apr;384(3):219–229. doi: 10.1007/BF00584556. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol. 1982 Dec;333:637–653. doi: 10.1113/jphysiol.1982.sp014473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Slow amphibian muscle fibres become less sensitive to Ca2+ with increasing sarcomere length. Pflugers Arch. 1983 May;397(3):248–250. doi: 10.1007/BF00584366. [DOI] [PubMed] [Google Scholar]
- Takagi A., Endo M. Guinea pig soleus and extensor digitorum longus: a study on single-skimmed fibers. Exp Neurol. 1977 Apr;55(1):95–101. doi: 10.1016/0014-4886(77)90161-3. [DOI] [PubMed] [Google Scholar]
- Wendt I. R., Stephenson D. G. Effects of caffeine on Ca-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflugers Arch. 1983 Aug;398(3):210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]


