Figure 2.
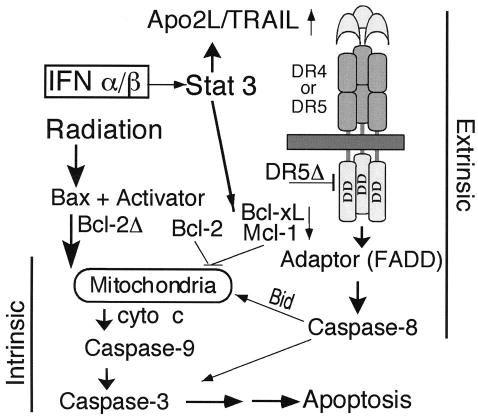
Model for activation of apoptosis in multiple myeloma by interferons (IFNs). After transcriptional induction by IFNs, Apo2 ligand (Apo2L) engages its death receptor 5 (DR5) or DR4 and through an adaptor Fas-associated death domain (FADD) recruits caspase 8 to the cell membrane, which can be blocked by a dominant-negative DR5Δ. After caspase 8 activation by proteolysis, Bid is cleaved and translocates to mitochondria, causing release of low levels of cytochrome c (cyto c) into the cytosol, a process that leads to caspase 9 and 3 activation. This process results in attack of the antiapoptotic protein Bcl-2 on the mitochondrial membranes, producing a truncated Bcl-2Δ protein, which causes release of more cyto c, caspase activation, and apoptosis. Bcl-xL or Mcl-1 transcriptional down-regulation mediated by signal transducers and activators of transcription 3 (Stat 3) is an additional mechanism by which IFNs may decrease levels of antiapoptotic proteins and shift the balance toward a proapoptotic state. (Modified from [6], with permission.) TRAIL indicates tumor necrosis factor–related apoptosis-inducing factor ligand; DD, death domain.
