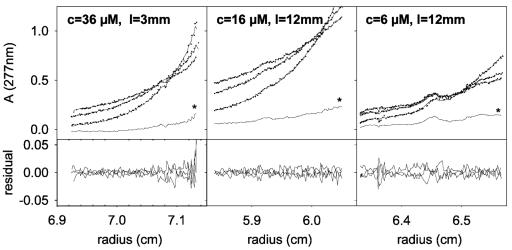
FIG. 4.
Equilibrium sedimentation of CHCV169*. CHCV169* was in C12E8 0.1%-150 mM NaCl-1 mM DTT-20 mM Tris-HCl (pH 8.0). Experiments were done for three protein concentrations c (36, 16, or 6 μM) using either 3- or 12-mm-path-length cells (I) as indicated in the corresponding panels. The equilibrium sedimentation profiles were recorded for three rotor velocities (13,000, 16,000, and 22,000 rpm from top to bottom at the origin). The data analysis was made considering an monomer-dimer equilibrium and mass conservation for each channel; the protein concentration at each position in the cell was calculated from the optical density. The top panels show the experimental data (•), the fit (continuous lines), and the calculated invariant time independent noise (★); the differences between the experimental and modeled profiles (residuals) are reported in the corresponding bottom panels. The resulting value for the buoyancy molar mass is close to that expected for the monomer polypeptide (120%), and that for the dissociation constant is 50 μM.