Abstract
A transgenic mouse model was used to identify an HLA-A*02-restricted epitope within the VP1 polypeptide of a human polyomavirus, BK virus (BKV), which is associated with polyomavirus-associated nephropathy in kidney transplant patients. Peptide stimulation of splenocytes from mice immunized with recombinant modified vaccinia virus Ankara expressing BKV VP1 resulted in expansion of cytotoxic T lymphocytes (CTLs) recognizing the sequence LLMWEAVTV corresponding to amino acid residues 108 to 116 (BKV VP1p108). These effector T-cell populations represented functional CTLs as assessed by cytotoxicity and cytokine production and were cross-reactive against antigen-presenting cells pulsed with a peptide corresponding to the previously described JC virus (JCV) VP1 homolog sequence ILMWEAVTL (JCV VP1p100) (I. J. Koralnik et al., J. Immunol. 168:499-504, 2002). A panel of 10 healthy HLA-A*02 human volunteers and two kidney transplant recipients were screened for T-cell immunity to this BK virus VP1 epitope by in vitro stimulation of their peripheral blood mononuclear cells (PBMC) with the BKV VP1p108 peptide, followed by tetramer labeling combined with simultaneous assays to detect intracellular cytokine production and degranulation. PBMC from 4/10 subjects harbored CTL populations that recognized both the BKV VP1p108 and the JCV VP1p100 peptides with comparable efficiencies as measured by tetramer binding, gamma interferon production, and degranulation. CTL responses to the JCV VP1p100 epitope have been associated with prolonged survival in progressive multifocal leukoencephalopathy patients (R. A. Du Pasquier et al., Brain 127:1970-1978, 2004; I. J. Koralnik et al., J. Immunol. 168:499-504, 2002). Given that both human polyomaviruses are resident in a high proportion of healthy individuals and that coinfection occurs (W. A. Knowles et al., J. Med. Virol. 71:115-123, 2003), our findings suggest a reinterpretation of this protective T-cell immunity, suggesting that the same VP1 epitope is recognized in HLA-A*02 persons in response to either BK or JC virus infection.
BK virus (BKV) is a ubiquitous human polyomavirus that causes asymptomatic primary infection and establishes persistent infection in several body sites, notably the kidney (5). In immunocompromised individuals, such as recipients of hematopoietic stem cell transplantation or kidney transplantation (KTx), BKV reactivation often occurs, as evidenced by viruria and/or viremia (16, 18, 19). In a minority of individuals, BKV can cause clinical disease, including hemorrhagic cystitis, ureteric stenosis, and BK virus-associated nephropathy (BKVN), which is associated with significant morbidity and mortality in KTx patients (29). The incidence of BKVN in KTx recipients has increased in recent years due to the use of newer and more potent immunosuppressive agents, suggesting that immune responses to BKV are important in control of the virus (18, 30). Current treatment options for BKVN are limited to reduction of immunosuppression and experimental use of cidofovir (20, 39). BK virus is closely related (∼70% homology) to a second human polyomavirus, JC virus (JCV), the etiologic agent of progressive multifocal leukoencephalopathy (PML), a fatal demyelinating disease of the central nervous system seen in immunocompromised patients with AIDS or cancer or recipients of organ transplantation (1). Two T-cell epitopes have been identified within the JCV major capsid protein VP1, and cytotoxic T lymphocytes (CTLs) recognizing these epitopes have been associated with control of the virus (12-14, 23).
Although limited investigations of T-cell immunity to BK virus have been described (6, 7, 9, 10), these studies employed BKV-infected cell lysates as complex antigens and did not define the specific antigens or epitopes recognized by the immune system. Knowledge of specific major histocompatibility complex class I-restricted epitopes within BK virus antigens could permit tracking of virus-specific CTLs in at-risk populations and analysis of the correlates of protective immunity to this increasingly important pathogen. We have employed transgenic (Tg) mice expressing human HLA-A*02 and infected with recombinant modified vaccinia virus Ankara (rMVA) expressing BKV antigens as a model system to analyze T-cell immune responses to BKV. We have also extended these studies to investigation of immune responses to BKV in healthy human volunteers and to KTx recipients.
MATERIALS AND METHODS
Study subjects.
Peripheral blood mononuclear cells (PBMC) were collected from 10 healthy HLA-A*02 donors at the City of Hope Comprehensive Cancer Center and from two HLA-A*02 KTx recipients at the University of Washington Medical Center. KTx recipient 4 (KTx04) had documented BKV viremia and viruria as measured by PCR, but no biopsy evidence of BKVN. KTx07 had documented viruria, but no viremia or biopsy evidence of BKVN. The study protocols were approved by the City of Hope and University of Washington Institutional Review Boards, and specimens and data were obtained prospectively after informed consent was obtained from the enrollees. HLA typing was performed by PCR, as described elsewhere (24) for all patients or blood donors.
Peptides.
Peptides were synthesized in our laboratory with a Symphony Quartet peptide synthesizer (Protein Technologies, Inc., Tucson, Arizona) using standard 9-fluorenylmethoxy carbonyl protocols and purified to >95% purity by high-performance liquid chromatography. The identities of the peptides were confirmed by matrix-assisted laser desorption ionization-time of flight mass spectrometric analysis using a Kompact Probe mass spectrometer (Kratos Analytical, Shimadzu Corp., Kyoto, Japan).
Tetramer construction.
The HLA-A*02 BKV VP1p108 (BKV VP1 with the sequence LLMWEAVTV corresponding to amino acid residues 108 to 116) and HLA-A*02 JCV VP1p100 (peptide corresponding to the previously described JCV VP1 homolog sequence ILMWEAVTL) tetramers were refolded, purified, and conjugated to fluorochromes in our laboratory using previously described methods (27).
Construction of rMVAs expressing BKV VP1.
Nucleotide sequences corresponding to the BKV VP1 open reading frame (ORF) sequence was cloned by PCR amplification using as a template a genomic clone of the MM strain of BKV (pBKV 33-1 [ATCC 45024]) obtained from the American Type Cell Culture Collection (43). The amplification product was cloned into pCR2.1 and verified by nucleotide sequencing before recloning into the recombination vector pLW22. Generation and selection of rMVA were performed using previously described methods (41). Verification of expression was done by immunostaining of BHK monolayers infected with the rMVA with an antipolyomavirus antibody (Novocastra).
Immunization of Tg mice and in vitro expansion of CTLs.
The transgenic mouse strain HHDII was obtained from F. Lemonnier (Pasteur Institute) (15). This mouse expresses a transgenic monochain histocompatibility class I molecule in which the C terminus of the human β2m is covalently linked to the N terminus of a chimeric heavy chain (HLA-A*0201-α1, -α2, H-2Db-α3-transmembrane and intracytoplasmic domains). Eight- to 12-week-old mice were immunized intraperitoneally with 3 × 107 to 5 × 107 PFU of rMVAs expressing BKV VP1. The animals were sacrificed after 2 weeks and the spleens retrieved. Single-cell splenocyte suspensions were prepared by passing the cells through a 70-μm Falcon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ) using the plunger from a sterile 1-ml syringe. Splenocytes were subjected to one round of in vitro expansion as previously described (27). Briefly, splenocytes from immunized animals were cocultured with peptide-loaded lipopolysaccharide blasts in complete in vitro stimulation medium at a ratio of 3:1 for 7 days, with the addition of 10% rat T-stim (Collaborative Biomedical Products, Bedford, MA).
Cytotoxicity assay.
Cytolytic activity of effector cell populations was determined using a 4-h chromium release assay following one round of in vitro stimulation, as previously described (8, 26). Jurkat A2.1 cells pulsed with 10 μM of the relevant or control human immunodeficiency virus peptides were used as targets for the chromium release assay. Jurkat A2.1 cells were loaded with 200 μCi of Na51 CrO4− (ICN, Costa Mesa, CA) for 1 h in a 37°C water bath and further processed as described previously (26). Experimental evaluations were performed in triplicate.
Intracellular cytokine assays.
Splenocytes after 1 week of in vitro stimulation were tested for intracellular gamma interferon (IFN-γ) production by stimulation overnight with 5 μM BKV VP1p108 or JCV VP1p100 peptide. The following day, brefeldin A was added to all the cultures and incubation continued for 4 h. The cells were then washed with 3 ml phosphate-buffered saline containing 0.5% bovine serum albumin (PBS-0.5% BSA) before they were labeled for 20 min at 4°C with fluorescein isothiocyanate (FITC)-conjugated antibody to murine CD8 (Pharmingen). The cells were then washed again with PBS-0.5% BSA before permeabilization (Cytofix/Cytoperm; Pharmingen) and labeling with allophycocyanin (APC)- or phycoerythrin (PE)-conjugated antibody to IFN-γ for 30 min at 4°C. The cells were washed and analyzed on a FACSCanto flow cytometer (Becton Dickinson).
In vitro stimulation of PBMC.
Cryopreserved human PBMC were cultured in 24-well tissue culture plates at a density of 3.5 × 106/ml in RPMI 10 medium containing 1 μg/ml of either BKV VP1p108 or JCV VP1p100 peptide at 37°C in a CO2-gassed incubator. After 3 days, recombinant human IL-2 (National Institutes of Health [NIH] AIDS Research and Reference Reagent Program) was added to 30 units/ml. Each 2 days thereafter, 50% of the culture medium was removed and replaced by fresh medium containing recombinant IL-2. Incubation was continued for 11 to 14 days before flow analysis.
Combined intracellular cytokine and CD107 mobilization/degranulation assay.
This combined intracellular cytokine and CD107 mobilization/degranulation assay was performed essentially as previously described (3). Cells from in vitro stimulation cultures were washed once with RPMI 10 medium. Aliquots of 1 × 106 cells were labeled with tetramers in 100 μl of medium for 30 min at 37°C. RPMI 10 medium (1 ml) and FITC-conjugated antibodies to CD107a and CD107b (Pharmingen) were then added to each aliquot, followed by the addition of costimulatory antibodies to CD28 and CD49d (Pharmingen) to 1 μg/ml each. Antigenic peptides corresponding to those incorporated in the tetramers or irrelevant peptides as controls were then added to some of the tubes to a final concentration of 5 μΜ. Monensin (1 μl) (GolgiStop; Pharmingen) was added to all the tubes before incubation at 37°C in a gassed incubator for 5 h. The cells were then washed with 3 ml PBS-0.5% BSA before labeling for 20 min at 4°C with PerCP (PhycoLink activated peridinin-chlorophyll-protein complex)-conjugated antibody to CD8 (Pharmingen). The cells were then washed again with PBS-0.5% BSA before permeabilization (Cytofix/Cytoperm; Pharmingen) and labeling with APC-conjugated antibody to IFN-γ (Pharmingen) for 30 min at 4°C. The cells were washed a final time and resuspended in 0.5 ml sheath fluid for flow analysis. A primary gate was set on lymphocytes using forward and side scatter, and a secondary gate was set on CD8+ tetramer-binding cells. At least 100,000 events were collected per sample. The percentage of CD8+ tetramer-binding lymphocytes expressing elevated surface CD107a/b and secreting IFN-γ was determined by reference to controls incubated with costimulatory antibodies to CD28 and CD49d but no peptides.
RESULTS
Prediction of HLA-A*02-restricted T-cell epitopes within BKV VP1 polypeptide and evaluation in cell culture and a Tg mouse model system.
A panel of six candidate T-cell epitopes (Table 1) were identified within the ORF encoding the BKV VP1 major capsid polypeptide using computer-based algorithms (SYFPEITHI, BIMAS, SVMHC, and FRAGPREDICT) that predict 9- or 10-mer amino acid sequences likely to be generated by proteasomal cleavage and to bind to HLA-A*02. Peptides corresponding to these sequences were tested for their ability to bind HLA-A*02 and stabilize its expression on the surfaces of T2 cells deficient in the transporter associated with antigen processing. The levels of surface HLA-A2 were measured by staining the peptide-pulsed cells with a fluorescently conjugated antibody to HLA-A2 and flow analysis. Five of the six VP1 peptides (the exception being the p109-118 peptide) clearly showed positive HLA-A2 binding as measured by an increase in relative fluorescence intensity on the surfaces of the cells (data not shown).
TABLE 1.
Candidate HLA-A2-restricted epitopes from BKV VP1
| Positions | Sequence |
|---|---|
| 26-35 | KLLIKGGVEV |
| 27-36 | LLIKGGVEVL |
| 27-35 | LLIKGGVEV |
| 108-116 | LLMWEAVTV |
| 107-116 | NLLMWEAVTV |
| 109-118 | LMWEAVTVQT |
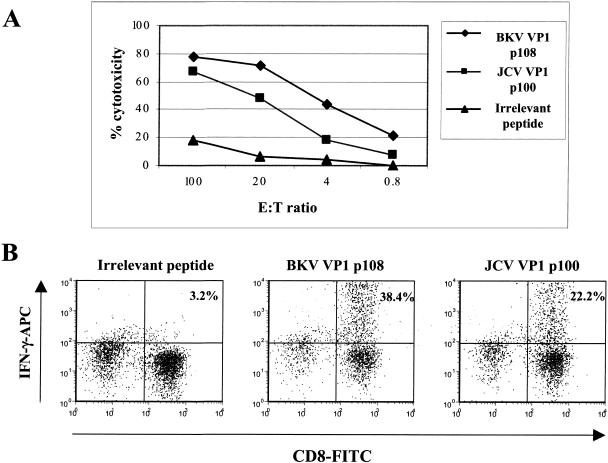
A transgenic murine model was then used to test whether peptides that showed positive HLA-A2 binding were generated by in vivo cellular processing of a full-length antigen expressed in MVA used for immunization. HHD-II transgenic mice were immunized intraperitoneally with rMVA expressing BKV VP1. Expression of the BKV polypeptide from this rMVA (a recombinant modified vaccinia virus Ankara expressing BKV VP1) was verified by immunostaining of infected BHK monolayers (not shown). Two weeks following immunization, the mice were sacrificed, the spleens were harvested, and the splenocytes were stimulated for 2 weeks in culture with the individual VP1 peptides. Following in vitro stimulation, immune splenocytes were tested for their abilities to lyse Jurkat A2 cells pulsed with cognate peptides and to produce IFN-γ upon peptide stimulation in intracellular cytokine assays. The results of these experiments indicated that only peptides VP1p108-116 and VP1p107-116 were recognized. The shorter VP1p108-116 peptide, LLMWEAVTV, likely represents the minimal cytotoxic epitope and was designated BKV VP1p108. The JCV homolog, ILMWEAVTL, which differs at the C-terminal and N-terminal positions from the BKV VP1 sequence has been described as a functional HLA-A*02-restricted T-cell epitope in humans (12, 14, 23). We synthesized this peptide, designated JCV VP1p100, and compared it with the BKV VP1p108 peptide in cytotoxicity and intracellular cytokine assays using the murine effectors elicited by immunization with rMVA-BKV VP1 and expanded by stimulation with BKV VP1p108 (Fig. 1). Both the intracellular cytokine and the cytotoxicity results suggest that these Tg murine CTLs recognized both the BKV and JCV VP1 homologs by cross-reaction. Slightly higher cytotoxicity was seen towards target cells pulsed with the BKV peptide, and somewhat higher IFN-γ production was observed in response to stimulation with the BKV peptide.
FIG. 1.
Immune responses to BKV VP1p108 and JCV VP1p100 epitopes in transgenic mice immunized with rMVA expressing BKV VP1. Transgenic HHD-II mice (four per group) were immunized intraperitoneally with 3 × 107 PFU of rMVA-BKV VP1. Two weeks after immunization, the mice were sacrificed, the spleens were harvested, and the splenocytes were cocultivated with syngeneic irradiated peptide-pulsed naïve mouse splenocytes. After 1 week, the cultures were tested for specific cytotoxicity versus A2-Jurkat cells pulsed with peptides (A) and in intracellular cytokine assays for IFN-γ production on peptide stimulation (B). E:T ratio, effector-to-target cell ratio; CD8-FITC, FITC-conjugated antibody to murine CD8; IFN-γ-APC, APC-conjugated antibody to IFN-γ.
Detection of CTL populations recognizing the BKV VP1p108 sequence among PBMC from HLA-A*0201 subjects.
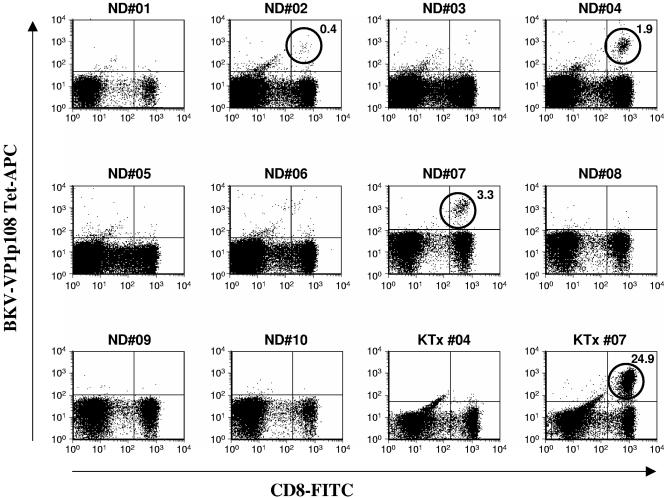
We were interested in determining whether cross-reactivity between two polyomavirus VP1 epitopes in Tg mice would also be seen in the context of human lymphocytes. To facilitate this analysis, we prepared an HLA-A*0201 tetramer incorporating the BKV VP1p108 peptide. This tetramer (BKV VP1p108 Tet-APC) was used to screen PBMC samples from 10 healthy donors and 2 kidney transplant recipients expressing the HLA-A*0201 phenotype. To enrich for CD8+ effector cells, the PBMC samples were stimulated for 2 weeks in culture with the VP1p108 peptide in the presence of IL-2 before tetramer analysis. The results, shown in Fig. 2, indicated that after this in vitro expansion PBMC from 2 of the 10 healthy donors (healthy or normal donor 4 [ND04] and ND07) had distinct populations of CD8+ T lymphocytes that bound BKV VP1p108 tetramer. Two more individuals (ND02 and ND06) may also have had very small tetramer-binding populations that were difficult to resolve from the assay background. The samples from one of two kidney transplant recipients tested harbored a large population of BKV VP1p108 tetramer-binding cells after stimulation. The frequency of these cells was 10-fold higher than that seen in the two positive healthy donors (24.9% of CD8+ T cells after amplification versus 1.9% and 3.3%). Labeling of unstimulated PBMC from donors ND04 and ND07 with the BKV VP1p108 tetramer did not detect any specific binding above background (data not shown), indicating that the levels of these CTL precursors in PBMC were below the detection limit of the tetramer-binding assay (approximately 0.05% of CD8+ PBMC).
FIG. 2.
Screening PBMC from healthy donors and KTx recipients for CTLs recognizing the BKV VP1p108 epitope. PBMC from 10 healthy donors (ND01 to ND10) and from two KTx recipients expressing the HLA-A*02 haplotype were stimulated once in culture with the BKV VP1p108 peptide in the presence of 30 U/ml recombinant IL-2. After a 14-day incubation period, the cultures were stained with FITC-conjugated antibody to CD8 (CD8-FITC) and with BKV VP1p108 Tet-APC before flow analysis. All plots are gated on lymphocytes by forward versus side scatter. Probable tetramer-binding populations are circled for emphasis, and their frequencies are indicated as a percentage of CD8+ T lymphocytes.
Functionality and specificity of the CD8+ T-cell population labeled by the BKV VP1p108 tetramer.
To address this question of functionality and specificity, we employed a modification of a recently described flow-based assay (3, 4, 42) that combines tetramer binding, intracellular cytokine reactivity, and measurement of CD107 binding. We have previously used a simpler version of this assay to investigate the functionality of cytomegalovirus-specific T cells (25). PBMC are first stained with tetramer and then stimulated for 4 h in culture with peptide in the presence of costimulatory antibodies and fluorescently conjugated antibodies to the lysosome-associated membrane proteins LAMP-1 (CD107a) and LAMP-2 (CD107b), present on the membranes of the cytotoxic granules. Monensin is also added to the culture to inhibit secretion of cytokines and to neutralize the pH within the cytotoxic granules that would quench fluorescence of fluorochrome conjugated to the CD107a and CD107b antibodies. If the cells recognize the peptide, then during this incubation step, engagement of the T-cell receptor by the complex formed by the peptide and major histocompatibility complex class I molecule will result in production of IFN-γ, mobilization of the cytotoxic granules to the cell surface, fusion with the plasma membrane, and exposure of the CD107a and CD107b markers to the exterior milieu to be labeled by the fluorescently conjugated antibodies to these markers. The cells are then fixed, permeabilized, and stained with fluorescently conjugated antibodies to IFN-γ and to CD8 before flow analysis (3).
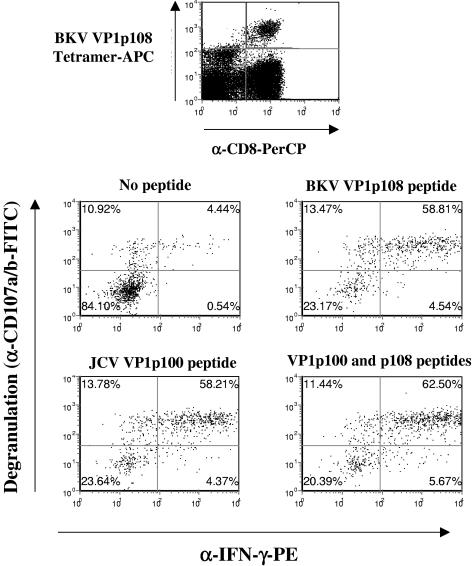
Figure 3 shows the result of a representative experiment on PBMC from ND07. Similar results were obtained with PBMC from ND04 (data not shown). Stimulation in culture with either the BKV VP1p108 or JCV VP1p100 peptide induced IFN-γ secretion and degranulation by the majority (58% or 59%) of the BKV VP1p108 tetramer-binding CD8+ T cells. In this assay, we observed a noticeable background (approximately 11%) of tetramer-binding cells with nonspecific degranulation and a smaller proportion of cells (approximately 4.5%) with both degranulation and IFN-γ production. This background may be due to the analysis being performed on PBMC that had been stimulated for 2 weeks in culture, since less background is seen with unstimulated PBMC (25). Stimulation with a combination of the two peptides only slightly increased the proportion of cells that degranulated and produced IFN-γ, indicating a high degree of overlap between the cells responding to the BKV VP1p108 peptide and those responding to the JCV VP1p100 peptide (Fig. 3). These experiments suggest that the majority of the CD8+ T cells bound by the BKV VP1p108 tetramer are functional CTLs, as assessed by mobilization of cytotoxic granules and cytokine production, and further, that they recognize both the BKV and JCV peptides.
FIG. 3.
Functionality of BKV VP1p108 tetramer-binding cells. PBMC from donor ND07 were expanded by in vitro stimulation with the BKV VP1p108 peptide in the presence of 30 U/ml recombinant IL-2. The expanded cell culture was then labeled with BKV VP1p108 Tet-APC and restimulated in culture for 4 h with peptides in the presence of costimulatory antibodies, monensin, and FITC-conjugated antibodies to CD107a and CD107b (α-CD107a/b-FITC) before permeabilization and labeling with a CyChrome-conjugated antibody to CD8 and a PE-conjugated antibody to IFN-γ (α-IFN-γ-PE). For more details, see Materials and Methods. For flow analysis, a primary gate was set on lymphocytes by forward versus side scatter, and in the case of the four CD107 versus IFN-γ plots, a primary gate was set on tetramer-positive cells. The values in the plot quadrants indicate cell numbers as a percentage of CD8+/BKV VP1p108 tetramer-positive lymphocytes.
Investigation of antigenic specificity of CD8+ T cells by simultaneous staining with BKV and JCV VP1 tetramers.
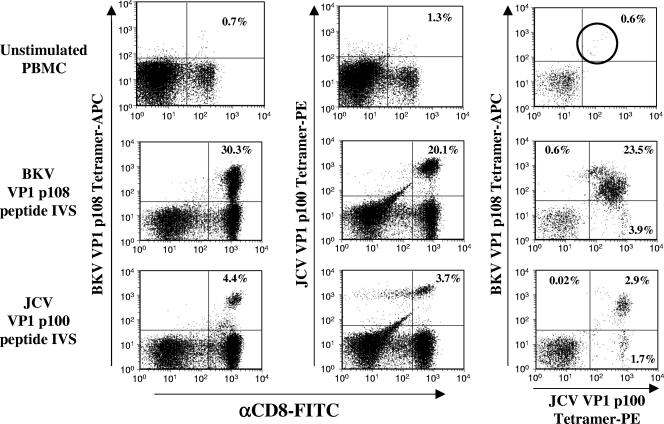
We tested CD8+ T cells expanded from human PBMC samples in response to stimulation with the BKV VP1p108 or JCV VP1p100 peptide for cross-recognition using VP1-specific tetramers. The JCV VP1p100 peptide was refolded into an HLA-A2 tetramer conjugated with the fluorescent molecule phycoerythrin. Since the BKV VP1p108 tetramer (Tet) was conjugated to allophycocyanin, this permitted the discrimination in flow cytometry between populations of cells binding either or both tetramers. We used PBMC from KTx07 for this experiment and stimulated aliquots of these cells in culture either with BKV VP1p108 peptide or with JCV VP1p100 peptide for 2 weeks in the presence of IL-2. Following this expansion, the cells were labeled with either BKV VP1p108 Tet-APC or JCV VP1p100 Tet-PE or with both tetramers together. For purposes of comparison, unstimulated PBMC from this subject were also labeled with these tetramers. The results are presented in Fig. 4 and show that in the absence of in vitro stimulation, the tetramer-binding cells were infrequent, close to assay background. However, double staining with the PE- and APC-conjugated tetramers assisted in the resolution of these cells (circled in the upper right-hand plot of Fig. 4) and permitted a more confident estimation of their frequency as approximately 0.6% of CD8+ lymphocytes in this unstimulated sample.
FIG. 4.
Costaining of PBMC and peptide-stimulated cell cultures from a KTx recipient with BKV and JCV VP1 tetramers. The top row of plots represent analyses performed on unstimulated, uncultured, cryopreserved PBMC from subject KTx07. The center row of plots represent analyses on an aliquot of these PBMC stimulated in culture with BKV VP1p108 peptide in the presence of recombinant IL-2. The bottom row represent analyses on an aliquot of these PBMC stimulated in culture with JCV VP1p100 peptide in the presence of recombinant IL-2. The cells were labeled with either BKV VP1p108 Tet-APC (left column) or JCV VP1p100 Tet-PE (center column) or both (right column). The values in the plot quadrants indicate tetramer-binding cell numbers as percentages of CD8+ lymphocytes. Plots in the right column represent analyses performed by gating on CD8+ lymphocytes. The double tetramer-positive population within the unstimulated PBMC is circled for emphasis. αCD8-FITC, FITC-conjugated antibody to murine CD8.
Stimulation with the BKV VP1p108 peptide (Fig. 4) expanded a sizeable population of tetramer-binding cells (30.3% binding the BKV tetramer and 20.1% binding the JCV tetramer). The majority (84%) of these tetramer-binding cells could simultaneously bind both the JCV and BKV tetramers (right plot of the middle row). Stimulation with the JCV VP1p100 peptide (Fig. 4) expanded a smaller population of tetramer-binding cells (4.4% binding the BKV tetramer and 3.7% binding the JCV tetramer). This difference in expansion efficiency could reflect a higher affinity of the CTL precursors within the PBMC for the BKV version of the epitope peptide. Again, a somewhat smaller majority (63%) of these tetramer-binding cells could simultaneously bind both the JCV and the BKV tetramers (right plot of the bottom row). This difference could reflect expansion in response to stimulation with the JCV peptide of a population of cells that bind the JCV tetramer but not the BKV tetramer.
We also investigated the effect of titration of the relative amounts of the two tetramers used to label these samples. Briefly, we found that increasing the amount of JCV VP1p100 tetramer while keeping the amount of BKV VP1p108 tetramer constant did not alter the proportion of the CD8+ cells binding either or both tetramer (data not shown). Thus, the results of these competitive tetramer-binding experiments did not alter our key finding of a high degree of cross-reactivity between the BKV and JCV variants of this T-cell epitope.
DISCUSSION
We have demonstrated that immunization of Tg mice with rMVA expressing BKV VP1 leads to the production of CTLs recognizing the BKV VP1p108 epitope LLMWEAVTV and cross-recognizing the JCV VP1 homolog sequence ILMWEAVTL. We have also shown that healthy seropositive HLA-A2 individuals and a KTx recipient also harbor low frequencies of CTL precursors that can be expanded by stimulation with either peptide into functional CTLs recognizing this BKV epitope and cross-recognizing the JCV homolog sequence. This HLA-A*02-restricted epitope is the first T-cell epitope that has been defined for BKV, offering the prospect of being able to use peptides and tetramers corresponding to this epitope to monitor immune responses to this virus in at-risk populations.
The results described in this report suggest a need to reappraise the data presented in a series of studies published by I. J. Koralnik, R. A. Du Pasquier, N. L. Letvin, and colleagues (13, 14, 23). These investigators identified the JCV VP1p100 epitope sequence by using a computer-based epitope prediction method similar to the method that we employed in this study (23). They used a peptide corresponding to this JCV VP1p100 epitope and a tetramer incorporating this peptide as tools to identify CTLs recognizing this epitope in HLA-A*02 human immunodeficiency virus-positive PML survivors (23) and in healthy individuals (14). Correlation of clinical PML status and the presence of CTLs recognizing this epitope was used to demonstrate an association between these cells and the early control of PML (13, 23). However, as we have demonstrated in the present study, the JCV VP1p100 epitope peptide and tetramer are not JCV specific, since they both cross-react with cells elicited in response to and recognizing the BKV homolog. Koralnik and colleagues did not report the BKV serostatus of their subjects. Several studies have shown that both JCV and BKV are common in most adult populations but that JCV is less prevalent than BKV (22, 31, 37). Knowles and colleagues reported 81% seropositivity for BKV and 35% seropositivity for JCV in a survey of 2,435 serum samples from 1991 (22). These types of studies are complicated by substantial serological cross-reactivities between antibodies to these two human polyomaviruses (40). However, antibody adsorption studies have shown that some individuals experience infection by both JCV and BKV (17), and this is further supported by PCR studies of polyomavirus shedding in urine that indicates coinfection in a minority of patients (2, 32, 36). Du Pasquier and colleagues also reported a second HLA-A2-restricted epitope within the JCV VP1 polypeptide (12, 14). This epitope, SITEVECFL, at position 36 within the ORF, differs by only the N-terminal amino acid from the BKV homolog AITEVECFL. It will be of interest to determine whether the latter sequence represents an immunodominant epitope of BKV and if so, whether a similar situation to JCV VP1p100/BKV VP1 p108 exists. We are currently addressing this question as part of our ongoing investigations into BKV immunity.
Our results indicate that T-cell responses recognizing the JCV VP1p100 epitope may have arisen from either JCV or BKV infection or both. The relative antigenicity of these two viruses in the context of this epitope may thus be influenced by their replication kinetics, tissue tropism, and other biological parameters. The significantly higher levels of BKV/JCV VP1-specific CD8+ T cells seen in one of two KTx recipients tested compared to the 10 healthy donors examined suggest the possibility that the documented BKV reactivation in this immunocompromised individual may have driven expansion of CTL precursors recognizing this epitope. We note that while this individual (KTx07) had documented viruria, viremia was not seen, suggesting more immune control over BKV replication than in subject KTx04, who experienced both viremia and viruria in the absence of detectable BKV VP1p108-specific T cells. Further investigation of T-cell immunity to BKV in a cohort of similar KTx recipients will be needed to address these issues, and this is currently in progress. It will be necessary to stringently determine which individuals in future study cohorts are infected with BKV and/or JCV using serological and/or PCR methods that can discriminate between the two viruses.
Given that these T-cell immune responses have been reported to protect against PML (11, 13, 23), it follows that prior BKV infection may cross-protect against JCV disease. Conversely, it may also imply that JCV infection could induce T-cell responses that might be protective against BKV-associated nephropathy. Many examples, mostly in murine models, of cross-reactive CTLs recognizing conserved epitopes encoded by families of related or even unrelated viruses have been described (for a recent review, see reference 34). CTLs recognizing a H-2kd-restricted epitope within the Ross River virus capsid cross-react with a range of other alphaviruses, including Sindbis and Semliki Forest viruses (28). Lymphocytic choriomeningitis virus-specific murine CTLs have been shown to be activated by infection with murine cytomegalovirus, vaccinia virus, or Pichinde virus (35). Similarly, cross-reactive, and in some cases, protective CTLs have been described for hantaviruses (38), influenza virus (33) and Epstein-Barr virus (21) subtypes. There is thus precedence for the possibility that CTL responses to one human polyomavirus could reduce the likelihood of infection and/or disease by the other virus.
These findings may have implications for the monitoring of at-risk populations for BKV and/or JCV disease and possibly for the design of immunointerventive therapies that could be targeted against both human polyomaviruses.
Acknowledgments
This work was supported by Public Health Service grant R21 CA104261 (S.F.L.), a supplement to 5PO1 CA30206 (S.F.L.), R01 CA77544 (D.J.D.), and P01 CA30206 (S.F.L.),of Project III (D.J.D.), all from the National Cancer Institute (NCI), and a Leukemia and Lymphoma Society Translational Research award R6145-05 (S.F.L.). The City of Hope Comprehensive Cancer Center is supported by the NCI (CA33572). The City of Hope General Clinical Research Center (GCRC; satellite of University of Southern California GCRC) is supported by the NIH (M01-RR043-38).
We thank Bernard Moss and Linda S. Wyatt (LVD, NIAID, NIH) for providing wild-type MVA and the pLW22 insertion plasmid. The transgenic HHDII mice were obtained from F. Lemonnier (Pasteur Institute). Constructs expressing HLA-A*0201 heavy chain and b2M were obtained from Patricia Roth (Beckman-Coulter). Human recombinant IL-2 was obtained from Maurice Gately, Hoffman-La Roche, Inc., through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. We thank Julia Santos and Donna Packer for administrative support, and we thank Stephen J. Forman and John Zaia for their support and encouragement.
REFERENCES
- 1.Antinori, A., A. Cingolani, P. Lorenzini, M. L. Giancola, I. Uccella, S. Bossolasco, S. Grisetti, F. Moretti, B. Vigo, M. Bongiovanni, B. Del Grosso, M. I. Arcidiacono, G. C. Fibbia, M. Mena, M. G. Finazzi, G. Guaraldi, A. Ammassari, M. A. D'Arminio, P. Cinque, and A. De Luca. 2003. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA). J. Neurovirol. 9(Suppl. 1):47-53. [DOI] [PubMed] [Google Scholar]
- 2.Bendiksen, S., O. P. Rekvig, M. Van Ghelue, and U. Moens. 2000. VP1 DNA sequences of JC and BK viruses detected in urine of systemic lupus erythematosus patients reveal no differences from strains expressed in normal individuals. J. Gen. Virol. 81:2625-2633. [DOI] [PubMed] [Google Scholar]
- 3.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. R., D. A. Price, J. M. Brenchley, K. Lore, F. J. Guenaga, A. Smed-Sorensen, D. R. Ambrozak, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2004. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J. Immunol. 172:6407-6417. [DOI] [PubMed] [Google Scholar]
- 5.Chesters, P. M., J. Heritage, and D. J. McCance. 1983. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J. Infect. Dis. 147:676-684. [DOI] [PubMed] [Google Scholar]
- 6.Comoli, P., A. Azzi, R. Maccario, S. Basso, G. Botti, G. Basile, I. Fontana, M. Labirio, A. Cometa, F. Poli, F. Perfumo, F. Locatelli, and F. Ginevri. 2004. Polyomavirus BK-specific immunity after kidney transplantation. Transplantation 78:1229-1232. [DOI] [PubMed] [Google Scholar]
- 7.Comoli, P., S. Basso, A. Azzi, A. Moretta, R. De Santis, F. Del Galdo, R. De Palma, U. Valente, A. Nocera, F. Perfumo, F. Locatelli, R. Maccario, and F. Ginevri. 2003. Dendritic cells pulsed with polyomavirus BK antigen induce ex vivo polyoma BK virus-specific cytotoxic T-cell lines in seropositive healthy individuals and renal transplant recipients. J. Am. Soc. Nephrol. 14:3197-3204. [DOI] [PubMed] [Google Scholar]
- 8.Daftarian, P., S. Ali, R. Sharan, S. F. Lacey, C. La Rosa, J. Longmate, C. Buck, R. F. Siliciano, and D. J. Diamond. 2003. Immunization with Th-CTL fusion peptide and cytosine-phosphate-guanine DNA in transgenic HLA-A2 mice induces recognition of HIV-infected T cells and clears vaccinia virus challenge. J. Immunol. 171:4028-4039. [DOI] [PubMed] [Google Scholar]
- 9.Drummond, J. E., K. V. Shah, and A. D. Donnenberg. 1985. Cell-mediated immune responses to BK virus in normal individuals. J. Med. Virol. 17:237-247. [DOI] [PubMed] [Google Scholar]
- 10.Drummond, J. E., K. V. Shah, R. Saral, G. W. Santos, and A. D. Donnenberg. 1987. BK virus specific humoral and cell mediated immunity in allogeneic bone marrow transplant (BMT) recipients. J. Med. Virol. 23:331-344. [DOI] [PubMed] [Google Scholar]
- 11.Du Pasquier, R. A., K. W. Clark, P. S. Smith, J. T. Joseph, J. M. Mazullo, U. De Girolami, N. L. Letvin, and I. J. Koralnik. 2001. JCV-specific cellular immune response correlates with a favorable clinical outcome in HIV-infected individuals with progressive multifocal leukoencephalopathy. J. Neurovirol. 7:318-322. [DOI] [PubMed] [Google Scholar]
- 12.Du Pasquier, R. A., M. J. Kuroda, J. E. Schmitz, Y. Zheng, K. Martin, F. W. Peyerl, M. Lifton, D. Gorgone, P. Autissier, N. L. Letvin, and I. J. Koralnik. 2003. Low frequency of cytotoxic T lymphocytes against the novel HLA-A*0201-restricted JC virus epitope VP1p36 in patients with proven or possible progressive multifocal leukoencephalopathy. J. Virol. 77:11918-11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Pasquier, R. A., M. J. Kuroda, Y. Zheng, J. Jean-Jacques, N. L. Letvin, and I. J. Koralnik. 2004. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain 127:1970-1978. [DOI] [PubMed] [Google Scholar]
- 14.Du Pasquier, R. A., J. E. Schmitz, J. Jean-Jacques, Y. Zheng, J. Gordon, K. Khalili, N. L. Letvin, and I. J. Koralnik. 2004. Detection of JC virus-specific cytotoxic T lymphocytes in healthy individuals. J. Virol. 78:10206-10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firat, H., F. Garcia-Pons, S. Tourdot, S. Pascolo, A. Scardino, Z. Garcia, M. L. Michel, R. W. Jack, G. Jung, K. Kosmatopoulos, L. Mateo, A. Suhrbier, F. A. Lemonnier, and P. Langlade-Demoyen. 1999. H-2 class I knockout, HLA-A2.1-transgenic mice: a versatile animal model for preclinical evaluation of antitumor immunotherapeutic strategies. Eur. J. Immunol. 29:3112-3121. [DOI] [PubMed] [Google Scholar]
- 16.Gardner, S. D., E. F. MacKenzie, C. Smith, and A. A. Porter. 1984. Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J. Clin. Pathol. 37:578-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton, R. S., M. Gravell, and E. O. Major. 2000. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. J. Clin. Microbiol. 38:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch, H. H., W. Knowles, M. Dickenmann, J. Passweg, T. Klimkait, M. J. Mihatsch, and J. Steiger. 2002. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N. Engl. J. Med. 347:488-496. [DOI] [PubMed] [Google Scholar]
- 19.Hogan, T. F., E. C. Borden, J. A. McBain, B. L. Padgett, and D. L. Walker. 1980. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann. Intern. Med. 92:373-378. [DOI] [PubMed] [Google Scholar]
- 20.Kadambi, P. V., M. A. Josephson, J. Williams, L. Corey, K. R. Jerome, S. M. Meehan, and A. P. Limaye. 2003. Treatment of refractory BK virus-associated nephropathy with cidofovir. Am. J. Transplant. 3:186-191. [DOI] [PubMed] [Google Scholar]
- 21.Kerr, B. M., N. Kienzle, J. M. Burrows, S. Cross, S. L. Silins, M. Buck, E. M. Benson, B. Coupar, D. J. Moss, and T. B. Sculley. 1996. Identification of type B-specific and cross-reactive cytotoxic T-lymphocyte responses to Epstein-Barr virus. J. Virol. 70:8858-8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowles, W. A., P. Pipkin, N. Andrews, A. Vyse, P. Minor, D. W. Brown, and E. Miller. 2003. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 71:115-123. [DOI] [PubMed] [Google Scholar]
- 23.Koralnik, I. J., R. A. Du Pasquier, M. J. Kuroda, J. E. Schmitz, X. Dang, Y. Zheng, M. Lifton, and N. L. Letvin. 2002. Association of prolonged survival in HLA-A2+ progressive multifocal leukoencephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J. Immunol. 168:499-504. [DOI] [PubMed] [Google Scholar]
- 24.Krausa, P., and M. J. Browning. 1996. A comprehensive PCR-SSP typing system for identification of HLA-A locus alleles. Tissue Antigens 47:237-244. [DOI] [PubMed] [Google Scholar]
- 25.Lacey, S. F., J. Martinez, G. Gallez-Hawkins, L. Thao, J. Longmate, W. Haq, R. Spielberger, S. J. Forman, J. A. Zaia, and D. J. Diamond. 2005. Simultaneous reconstitution of multiple cytomegalovirus-specific CD8+ cell populations with divergent functionality in hematopoietic stem-cell transplant recipients. J. Infect. Dis. 191:977-984. [DOI] [PubMed] [Google Scholar]
- 26.La Rosa, C., R. Krishnan, S. Markel, J. P. Schneck, R. Houghten, C. Pinilla, and D. J. Diamond. 2001. Enhanced immune activity of cytotoxic T-lymphocyte epitope analogs derived from positional scanning synthetic combinatorial libraries. Blood 97:1776-1786. [DOI] [PubMed] [Google Scholar]
- 27.La Rosa, C., Z. Wang, J. C. Brewer, S. F. Lacey, M. C. Villacres, R. Sharan, R. Krishnan, M. Crooks, S. Markel, R. Maas, and D. J. Diamond. 2002. Preclinical development of an adjuvant-free peptide vaccine with activity against CMV pp65 in HLA transgenic mice. Blood 100:3681-3689. [DOI] [PubMed] [Google Scholar]
- 28.Linn, M. L., L. Mateo, J. Gardner, and A. Suhrbier. 1998. Alphavirus-specific cytotoxic T lymphocytes recognize a cross-reactive epitope from the capsid protein and can eliminate virus from persistently infected macrophages. J. Virol. 72:5146-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nickeleit, V., H. H. Hirsch, I. F. Binet, F. Gudat, O. Prince, P. Dalquen, G. Thiel, and M. J. Mihatsch. 1999. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J. Am. Soc. Nephrol. 10:1080-1089. [DOI] [PubMed] [Google Scholar]
- 30.Nickeleit, V., H. H. Hirsch, M. Zeiler, F. Gudat, O. Prince, G. Thiel, and M. J. Mihatsch. 2000. BK-virus nephropathy in renal transplants—tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol. Dial. Transplant. 15:324-332. [DOI] [PubMed] [Google Scholar]
- 31.Padgett, B. L., and D. L. Walker. 1973. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J. Infect. Dis. 127:467-470. [DOI] [PubMed] [Google Scholar]
- 32.Priftakis, P., G. Bogdanovic, G. Tyden, and T. Dalianis. 2000. Polyomaviruria in renal transplant patients is not correlated to the cold ischemia period or to rejection episodes. J. Clin. Microbiol. 38:406-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambhara, S., S. Woods, R. Arpino, A. Kurichh, A. Tamane, B. Underdown, M. Klein, B. K. Lovgren, B. Morein, and D. Burt. 1998. Heterotypic protection against influenza by immunostimulating complexes is associated with the induction of cross-reactive cytotoxic T lymphocytes. J. Infect. Dis. 177:1266-1274. [DOI] [PubMed] [Google Scholar]
- 34.Selin, L. K., M. Cornberg, M. A. Brehm, S. K. Kim, C. Calcagno, D. Ghersi, R. Puzone, F. Celada, and R. M. Welsh. 2004. CD8 memory T cells: cross-reactivity and heterologous immunity. Semin. Immunol. 16:335-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selin, L. K., S. R. Nahill, and R. M. Welsh. 1994. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J. Exp. Med. 179:1933-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah, K. V., R. W. Daniel, H. D. Strickler, and J. J. Goedert. 1997. Investigation of human urine for genomic sequences of the primate polyomaviruses simian virus 40, BK virus, and JC virus. J. Infect. Dis. 176:1618-1621. [DOI] [PubMed] [Google Scholar]
- 37.Taguchi, F., J. Kajioka, and T. Miyamura. 1982. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol. Immunol. 26:1057-1064. [DOI] [PubMed] [Google Scholar]
- 38.Van Epps, H. L., C. S. Schmaljohn, and F. A. Ennis. 1999. Human memory cytotoxic T-lymphocyte (CTL) responses to Hantaan virus infection: identification of virus-specific and cross-reactive CD8+ CTL epitopes on nucleocapsid protein. J. Virol. 73:5301-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vats, A., R. Shapiro, R. P. Singh, V. Scantlebury, A. Tuzuner, M. Saxena, M. L. Moritz, T. J. Beattie, T. Gonwa, M. D. Green, and D. Ellis. 2003. Quantitative viral load monitoring and cidofovir therapy for the management of BK virus-associated nephropathy in children and adults. Transplantation 75:105-112. [DOI] [PubMed] [Google Scholar]
- 40.Viscidi, R. P., D. E. Rollison, E. Viscidi, B. Clayman, E. Rubalcaba, R. Daniel, E. O. Major, and K. V. Shah. 2003. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-like-particle-based enzyme immunoassays. Clin. Diagn. Lab. Immunol. 10:278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, Z., C. La Rosa, R. Maas, H. Ly, J. Brewer, S. Mekhoubad, P. Daftarian, J. Longmate, W. J. Britt, and D. J. Diamond. 2004. Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus. J. Virol. 78:3965-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolint, P., M. R. Betts, R. A. Koup, and A. Oxenius. 2004. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J. Exp. Med. 199:925-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, R. C., and R. Wu. 1979. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science 206:456-462. [DOI] [PubMed] [Google Scholar]