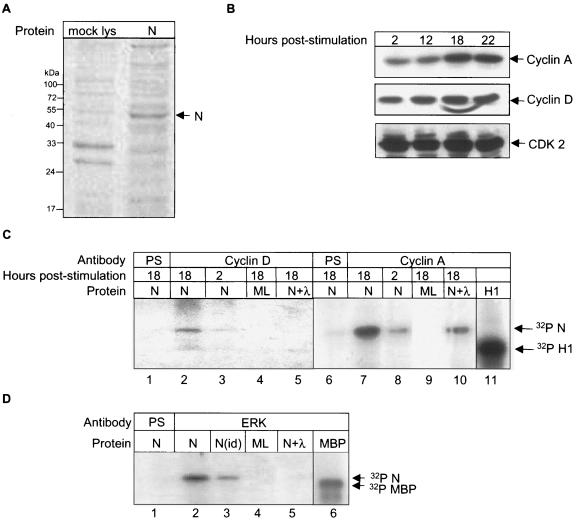
FIG. 5.
In vitro phosphorylation of the N protein by cyclin-CDK complex and mitogen-activated protein kinase. (A) Coomassie brilliant blue-stained gel showing expression of the N protein (lane 2) in an in vitro translation reaction. Lane 1 represents mock-translated lysate. (B) COS-1 cells were starved for 34 h in serum-free medium, stimulated with complete medium for the indicated times, and equal amounts of the lysates were Western blotted using cyclin A (upper panel), cyclin D (middle panel), and CDK2 (lower panel) antibodies. Bands were detected by the enhanced chemiluminescence method. (C) COS-1 cells were starved for 34 h in serum-free medium, stimulated with complete medium for the indicated periods, and immunoprecipitated using the respective antibodies followed by incubation with in vitro-expressed N protein (lanes 1, 2, 3, 5, 6, 7, 8, and 10) or mock-expressed lysate (ML; lanes 4 and 9). Lanes 1 and 6 were immunoprecipitated using preimmune serum (PS). Bands corresponding to in vitro-phosphorylated N were observed by autoradiography. Lanes 5 and 10 represents N protein treated with λ phosphatase. Lane 11 shows phosphorylated histone H1. Lane 11 is a gel exposed for a shorter period. (D) In vitro phosphorylation of N protein by ERK1/2. COS-1 cells were immunoprecipitated using preimmune serum (lane 1) or ERK1/2 antibody (lanes 2 to 5). Lane 3 represents ERK1/2 immunodepleted sample. Lanes 4 and 5 represent mock lysate (ML) and λ phosphatase-treated samples, respectively. Lane 6 shows the phosphorylated maltose-binding protein band. Lane 6 was exposed for a shorter period.