Abstract
Lymphangiogenesis is associated with pathological processes such as the metastatic spread of carcinoma cells and organization of immunologically active lymphocytic infiltrates following organ transplantation. It has not yet been established whether expansion of the lymphatic vascular meshwork is driven by incorporation of progenitor cells or by local endothelial cell division. In this issue of the JCI, Maruyama et al. provide evidence that after mouse corneal transplant, CD11b+ macrophages infiltrate the corneal stroma and transdifferentiate into lymphatic endothelial cell clusters that join existing lymphatic vessels. In complementary in vitro experiments, murine peritoneal macrophages expressed lymphatic endothelial markers and formed vessel-like protrusions. These findings add yet another facet to the plasticity of macrophages, which are already known to transform from naive monocytes into VEGF-C–producing cells. Thus, macrophages support lymphangiogenesis in 2 different ways, either by transdifferentiating and directly incorporating into the endothelial layer or by stimulating division of preexistent local lymphatic endothelial cells.
The molecular biology and pathology of lymphatic vessels and their endothelial cells has been one of the most rapidly growing fields of vascular biology in recent years. Historically, ancient anatomists visually recognized the larger vessels of the lymphatic system by their ability to collect intradermally injected tracers. About 40 years ago, the pioneering work of Leak and Burke established morphological differences between lymphatic and blood vessels, and the first attempts were made to culture lymphatic endothelial cells (1). However, as no specific marker proteins that were exclusively expressed by lymphatic endothelial cells had been discovered, characterization and tissue localization of lymphatics remained elusive. Some of the earliest landmark findings in this field are considered to be the discovery of the lymphatic endothelial cell–specific growth factor VEGF-C and its tyrosine kinase receptor, VEGFR-3 (2), which was followed by the discovery of podoplanin, a membrane mucoprotein expressed abundantly in lymphatic endothelial cells (3); the CD44-related hyaluronic acid receptor LYVE-1 (4); and the transcription factor PROX-1 (5), which presumably serves as a master switch for the lymphatic phenotype of endothelial cells (6, 7). With this knowledge it was possible to isolate pure lymphatic endothelial cells and grow them in tissue culture (8, 9) and to precisely localize lymphatics in tissues in health and disease.
Our rapidly evolving understanding of the basic aspects of lymphatic endothelial cell biology has prompted examination of the mechanisms of pathological lymphangiogenesis. In this issue of the JCI, Maruyama et al. (10) provide what is believed to be the first direct evidence that, following mouse corneal transplantation, CD11b+ macrophages are able to transdifferentiate into lymphatic endothelial cells. This finding represents an important milestone in our understanding of the contribution of cells of the innate immune system to pathological lymphangiogenesis.
How do lymphatic vessels grow?
Extension of vessels occurs by 2 alternative mechanisms: mitosis of endothelial cells and vascular sprouting or appositional growth involving integration of endothelial progenitor cells at sites of extension. In the blood vasculature, evidence for the occurrence of both mechanisms was established in animal experiments; however, a major caveat against generalization of these results has been the lack of relevance of these findings to human diseases. While a more realistic picture of the mechanisms of angiogenesis is now slowly emerging from cumbersome studies on human tissues (11), very little is known by comparison about the mechanisms of lymphangiogenesis. Previously, de novo lymphangiogenesis has been observed in mature mouse models following transgenic overexpression of VEGF-C in mouse basal keratinocytes (2) or in pancreatic islets (12, 13) and was also found to occur in and around human squamous epithelial carcinomas (14). An especially favorable location in which to study lymphangiogenesis by direct microscopy is the cornea (reviewed in ref. 15). While the normal cornea is devoid of blood and lymphatic vessels, both vessel types invade the cornea following corneal disease and surgery or transplant. In the context of the Maruyama et al. study (10), it is important to note that lymphangiogenesis occurs in the inflammatory setting of corneal transplantation, and the results of this study have now provided the first clue to my knowledge regarding the direct participation of macrophages in this process.
Macrophage transdifferentiation: the missing link in lymphangiogenesis
A cardinal question in adult lymphangiogenesis is whether endothelial cells in new vessels are derived from circulating progenitors or from local preexisting vessels by cell division and sprouting. The first indications of a role for local endothelial cell sprouting in lymphangiogenesis were derived from elegant experiments in which sublethally irradiated mice were grafted with GFP-expressing bone marrow (16). After induction of dermal lymphatic vessel proliferation, it was found that not a single lymphatic endothelial cell was derived from the donor’s bone marrow. By contrast, there are also some indications of the existence of circulating lymphatic endothelial progenitor cells, which constitutively express VEGFR-3 on their surface and express lymphatic endothelial cell–specific proteins in culture. One potential lymphatic progenitor lineage was isolated as a minor subtraction from human hemangioblast preparations (17). Remarkably, however, a major proportion of human circulating CD14+ naive monocytes also constitutively expressed VEGFR-3, and it was speculated that these cells are the source of VEGF-C–expressing tissue macrophages in the tumor stroma (14) and in inflammatory infiltrates in transplanted kidney and cornea (18, 19). In their present study (10), Maruyama and colleagues provide the first evidence to my knowledge that macrophages transdifferentiate in a stepwise fashion into lymphatic endothelial cells, initially forming cell aggregates that presumably develop into morula-like vesicles and then integrate into sprouting lymphatic vessels. This was confirmed by the authors’ finding that ablation of macrophages by treatment with clodronate liposomes completely abolished corneal lymphangiogenesis. Moreover, under appropriate in vitro culture conditions, activated murine peritoneal CD11b+ macrophages formed tube-like structures and expressed some lymphatic endothelial cell–specific markers. Taken together, these data provide strong evidence that CD11b+ macrophages possess the capacity to transdifferentiate into lymphatic endothelial cells.
However, macrophages appear to also play an alternative role in lymphangiogenesis, in that a subpopulation of these cells is reprogrammed to produce large amounts of VEGF-C and thus induce local sprouting of preexisting lymphatic endothelial cells. The first suggestion of such a mechanism followed the observation that activated murine macrophages express VEGFR-3 and are chemotactically attracted by VEGF-C in vitro (20), which was then followed by the observation that a subset of peritumoral-activated macrophages express VEGF-C and presumably contribute to peritumoral lymphatic vessel expansion (14). Subsequently, VEGF-C–producing macrophages were found to participate in lymphangiogenesis in human renal transplant rejection (19) and in the mouse trachea after induction of inflammation by inoculation with tubercle bacteria (21). It remains to be established which factors determine the fate and regulate the differentiation of tissue macrophages, be they involved in the induction of preexisting endothelial cell sprouting or transdifferentiation into lymphatic endothelial cells.
Lymphatic vessel function revisited
Given all the efforts in the last few years to gain further insight into lymphatic endothelial biology and to better understand the molecular mechanisms of lymphangiogenesis, the question arises as to whether these investments are justified in view of recent insights into lymphatic vessel function. The traditionally acknowledged functions of lymphatic vessels include collection of interstitial tissue fluid and cells, drainage to the next lymph node for “immunological inspection,” and recirculation to the venous blood system via the thoracic duct. Recent data suggest that these functions are not caused by a passive process but involve rapid and highly efficient transport (22). For a long time, it has been known that interruption of lymphatic fluid transport either by vascular malformations or surgical interventions resulted in lymphedema. Although lymphatic endothelial cells are endowed with caveolae and other cellular organelles that mediate endo- and/or transcytosis (8, 23), the traditional view persists that fluid and solutes enter the lymphatic lumen passively via the paracellular pathway through leaky intercellular junctions that differ in their molecular composition from those of blood vascular endothelial cells (8). Recently, evidence was provided that this transport pathway for solutes is of paramount importance not only for fluid homeostasis in normal tissues, but also for metastatic dissemination of tumors. Apparently, metastasis-competent primary tumors produce and secrete lymphoendotheliotrophic factors, which are collected by regional lymphatics and drained into the sentinel lymph node. Here they cause transformation of sinuses and/or genuine intranodal lymphangiogenesis as a prelude for adhesion of metastasizing tumor cells, thus substantiating the “seed and soil” hypothesis of tumor dissemination (24). Of interest is the interaction of inflammatory cells with lymphatic endothelium. This is obviously important in the migration of antigen-loaded dendritic cells or tumor cells that find their way to the next lymphatic vessel and transmigrate into the vascular lumen. The molecular mechanisms governing these processes are currently under investigation because targeted disruption of this pathway could be of therapeutic use. In the unique situation of lymphangiogenesis in renal transplants and the association of lymphatics with active lymphocytic infiltrates, a potential mechanism was delineated that involves secretion of the chemokines SLC/CCL21 by lymphatic endothelial cells and consequent attraction of CCR7+ lymphocytes and dendritic cells (19). Thus, it is possible that lymphatic endothelial cells orchestrate the formation of (tertiary) lymphatic organs. These selected examples highlight unanticipated, yet important, functions of lymphatic vessels that can now be unraveled with newly available molecular instruments, and it is safe to predict that surprises are still ahead of us.
Perspective
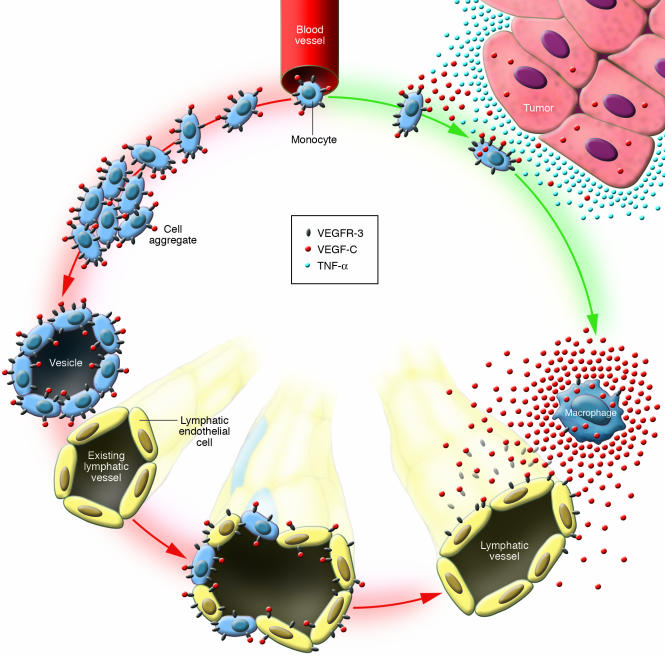
In summary, the novel data reported by Maruyama et al. (10) indicate that macrophages apparently play 2 different roles in lymphangiogenesis (Figure 1). They may serve as a source of VEGF-C and thus trigger growth or hyperplasia of lymphatic vessels through the sprouting of preexistent lymphatics. Alternatively, macrophages may transdifferentiate into lymphatic endothelial cells.
Figure 1.
Lymphangiogenesis: a split path for monocytes/macrophages. A subfraction of naive blood-borne monocytes constitutively express VEGFR-3 on their surface. It has been proposed that these monocytes emigrate from blood vessels and then follow 2 different pathways. Pathway A (green arrows): Monocytes are exposed to TNF-α and/or other proinflammatory agents in the (peritumoral) stroma and are converted into VEGF-C_ secreting macrophages that presumably induce proliferation of lymphatic endothelial cells. Pathway B (red arrows): In this issue of the JCI, Maruyama et al. (10) describe how, in a mouse corneal transplant model, macrophages transdifferentiate into lymphatic endothelial cells by forming cell aggregates and vesicles that integrate into an existing lymphatic vessel.
A hallmark of good research is that it opens a door to a new room with many other doors. In describing a novel role for tissue macrophages in lymphangiogenesis, Maruyama et al. (10) have done just that. One obvious emerging question prompted by this research is whether the concept of appositional growth of lymphatics and involvement of circulation-derived precursors is a general phenomenon or whether it is context-dependent and occurs, for example, only when rapid lymphangiogenesis takes place, as is the case with inflammation in the transplanted cornea. Also, the relation of these chameleon-like macrophages to circulating precursors such as CD14+ monocytes needs to be explored. Finally, as the ultimate goal of biomedical research is to learn about the pathogenesis of human diseases and to develop precisely targeted therapies, the contribution of macrophages to pathologic lymphangiogenesis needs to also be established in humans. As in many other instances, the elegant experiments performed here in mice (10) will undoubtedly provide an important landmark in the journey into the essentially uncharted territory of lymphatic vessel function in human disease.
Acknowledgments
The author is supported in part by the European Union 5th Framework Project “Chronic Kidney Disease” (QLG1-CZ-2000-00619) and 6th Framework Integrated Project “Lymphangiogenomics” (LSGH-2004-503573) and by a grant from the Center of Excellence in Oncology (CLEXO) at Medical University Vienna.
Footnotes
See the related article beginning on page 2363.
Conflict of interest: The author has declared that no conflict of interest exists.
References
- 1.Leak L, Burke J. Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am. J. Anat. 1966;118:785–810. doi: 10.1002/aja.1001180308. [DOI] [PubMed] [Google Scholar]
- 2.Jeltsch M, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 3.Breiteneder-Geleff S, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerji S, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 6.Wigle JT, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrova TV, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kriehuber E, et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makinen T, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maruyama K, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 2005;115:2363–2372. doi:10.1172/JCI23874. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters BA, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat. Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 12.Mandriota SJ, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skobe M, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 14.Schoppmann SF, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis. Cornea. 2003;22:273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 16.He Y, et al. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 17.Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 18.Cursiefen C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 2004;113:1040–1050. doi:10.1172/JCI200420465. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerjaschki D, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 20.Skobe M, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am. J. Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baluk P, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Invest. 2005;115:247–257. doi:10.1172/JCI200522037. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenstad O, Heyeraas KJ, Wiig H, Aukland K. Drainage of plasma proteins from the renal medullary interstitium in rats. J. Physiol. 2001;536:533–539. doi: 10.1111/j.1469-7793.2001.0533c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podgrabinska S, et al. Molecular characterization of lymphatic endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16069–16074. doi: 10.1073/pnas.242401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirakawa S, et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]