Abstract
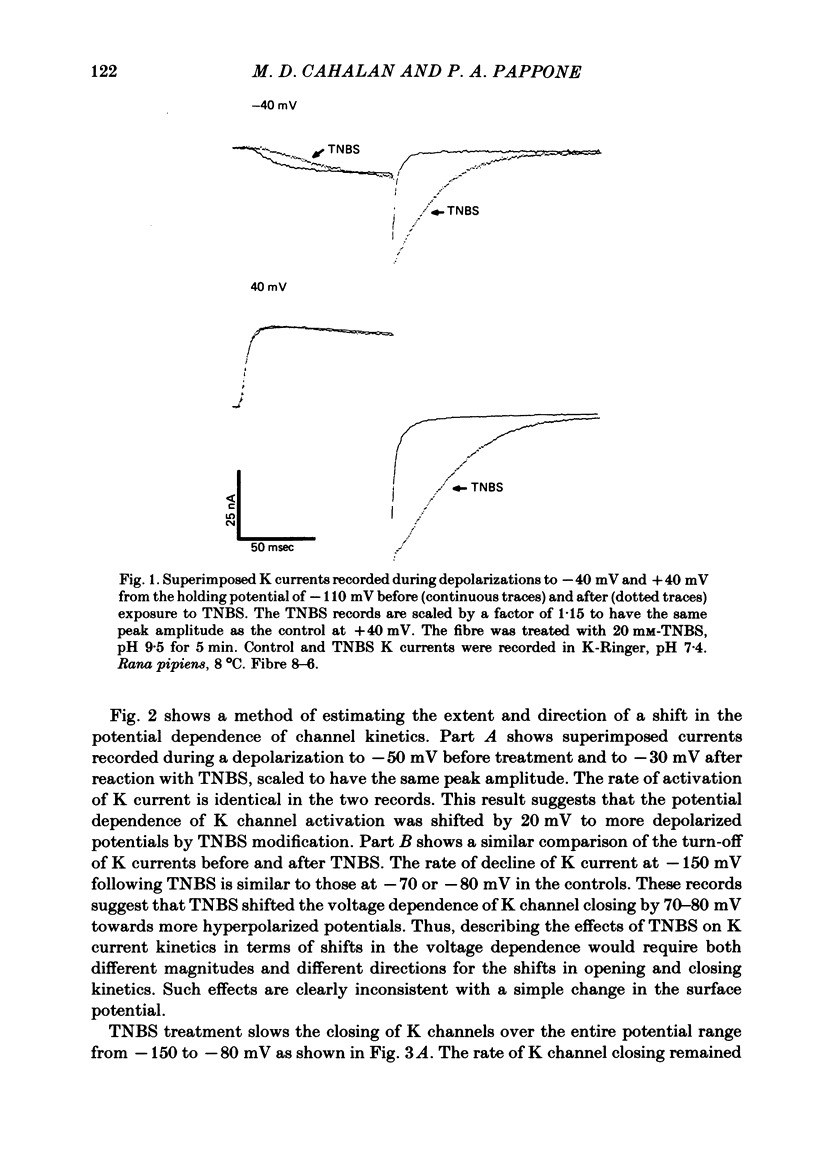
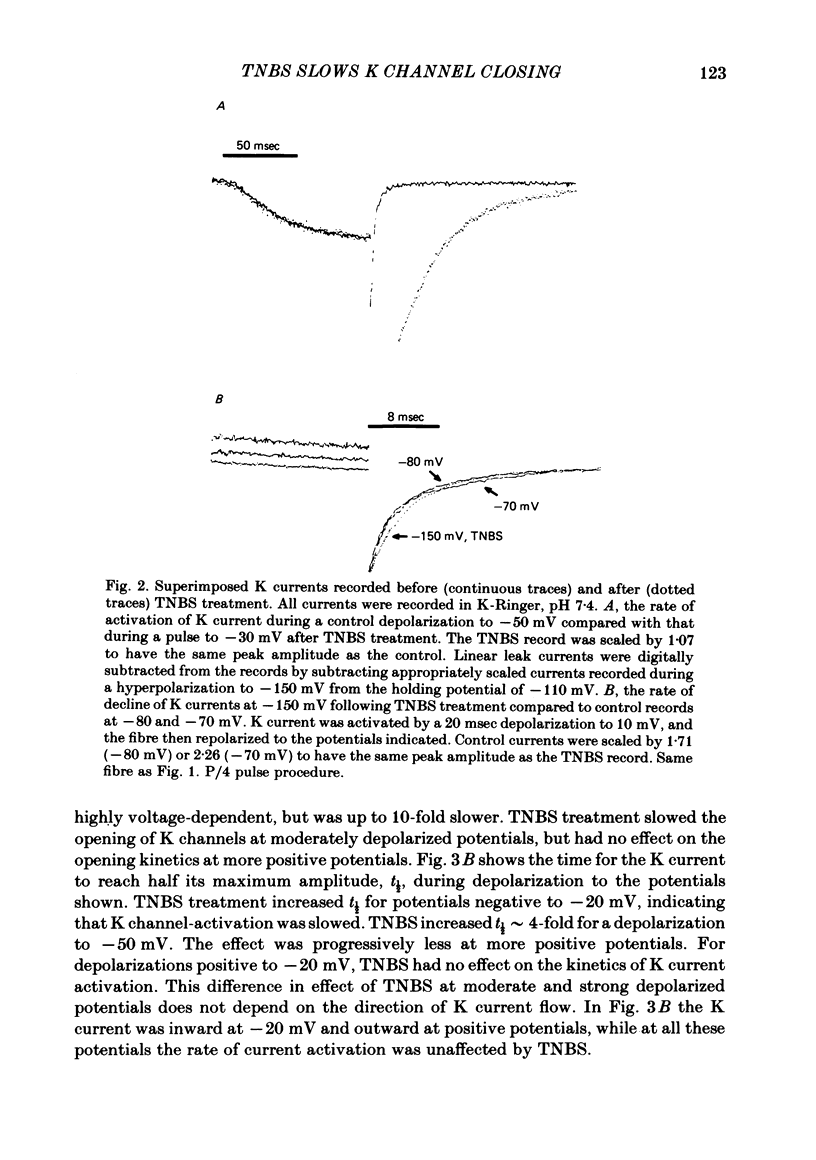
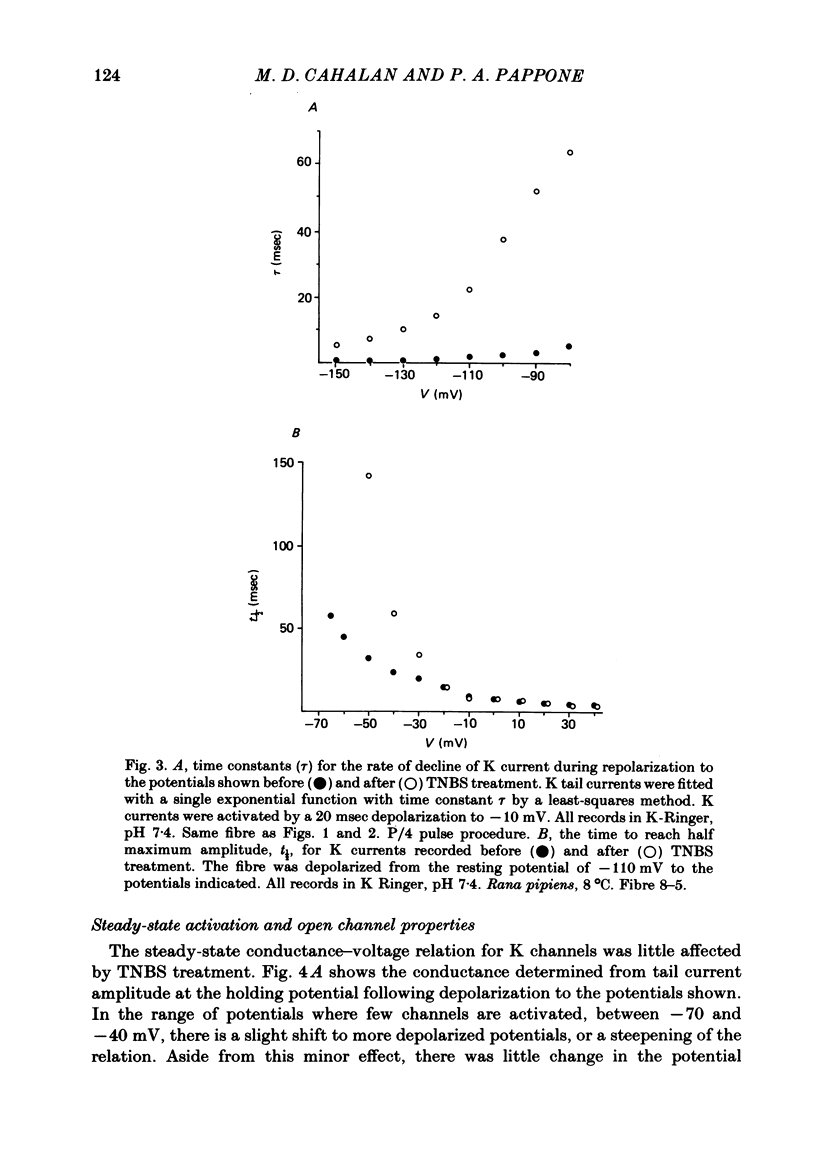
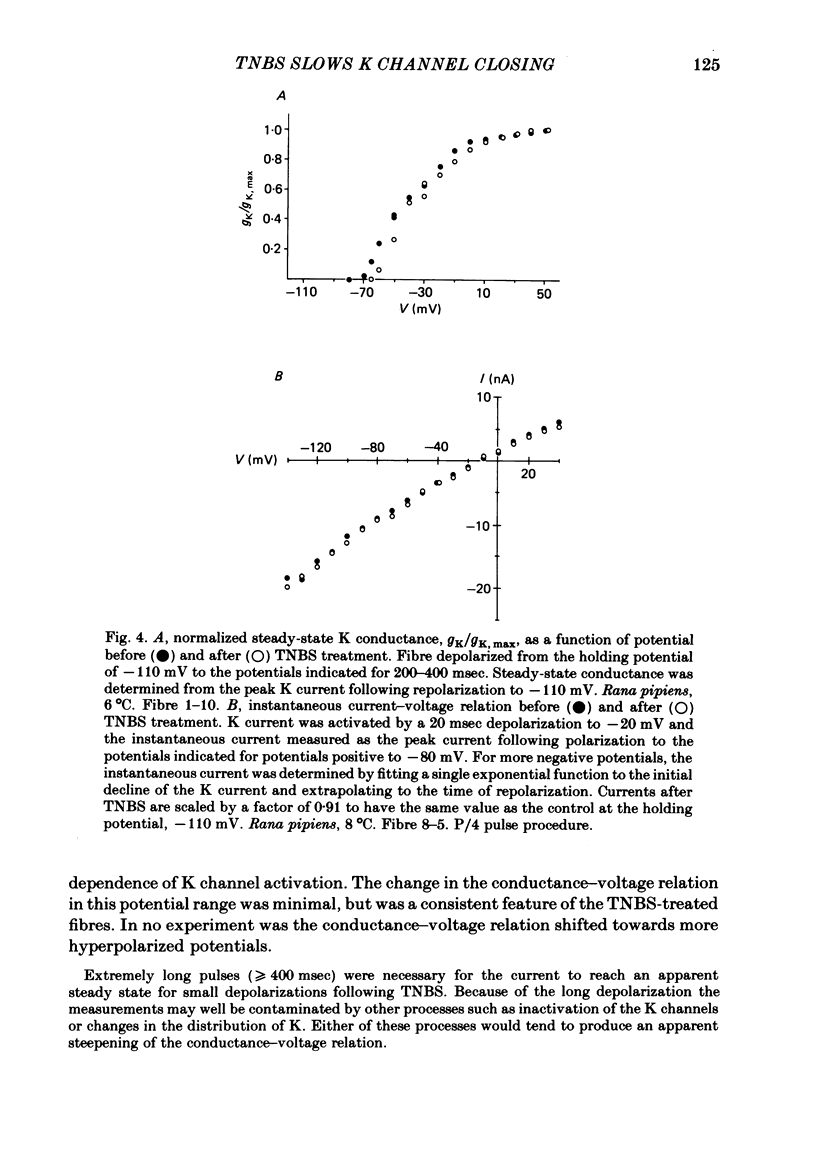
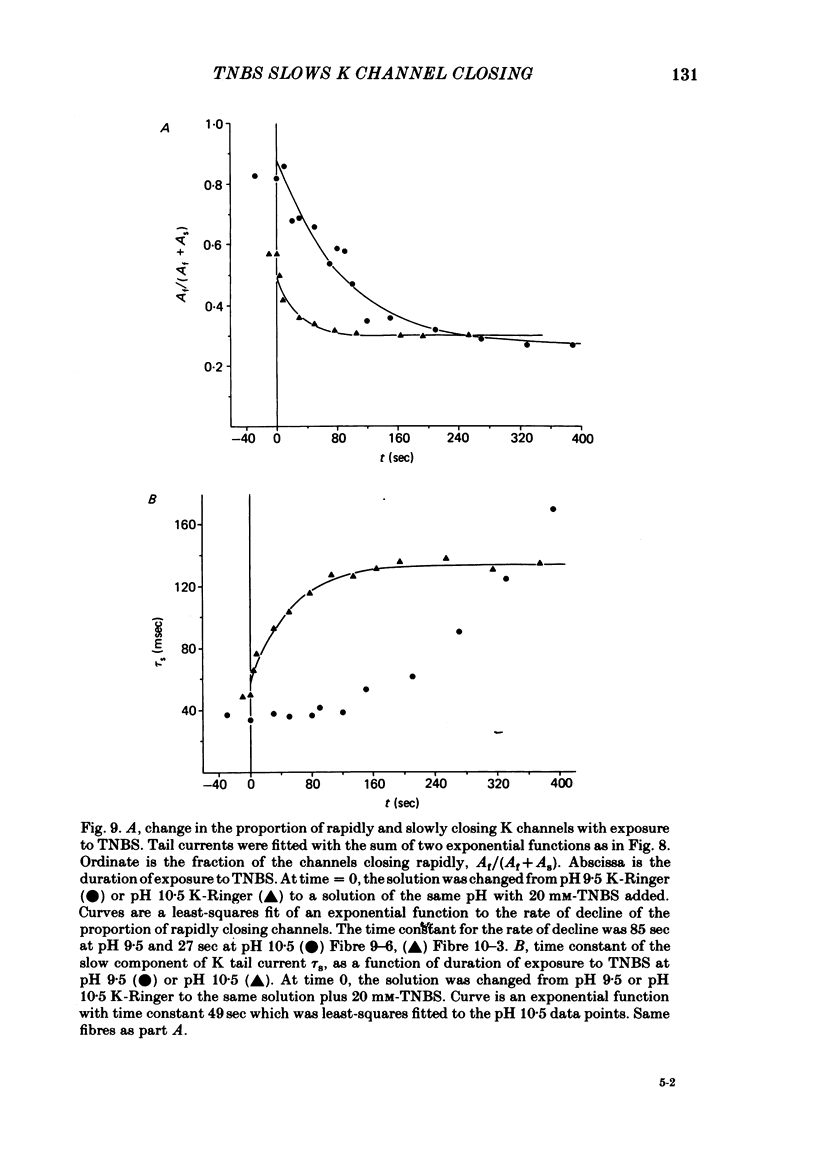
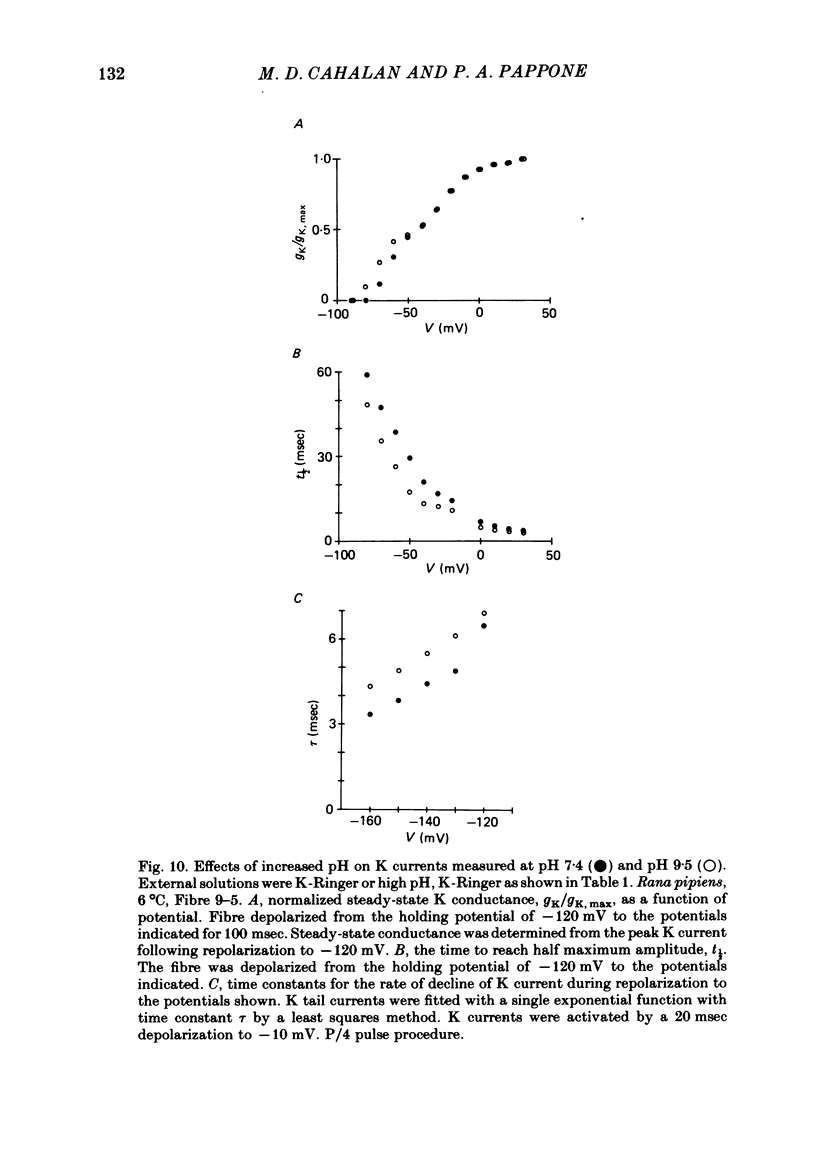
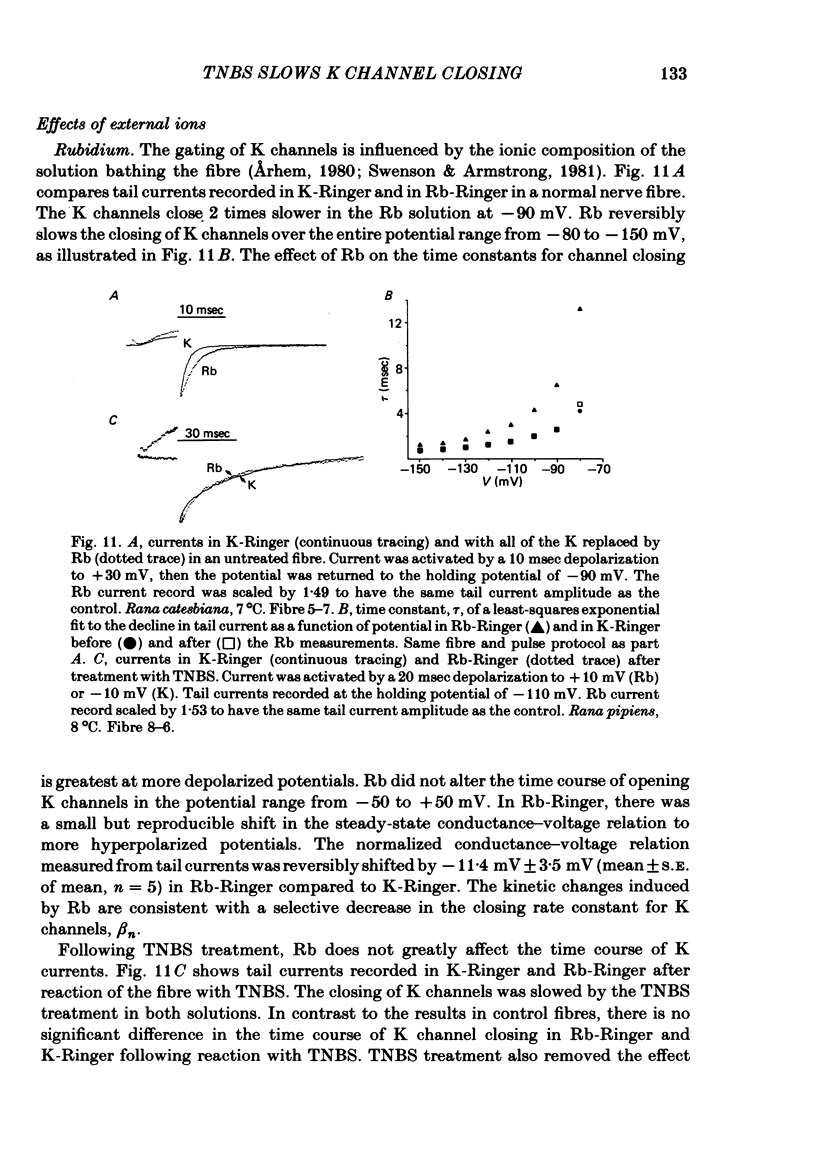
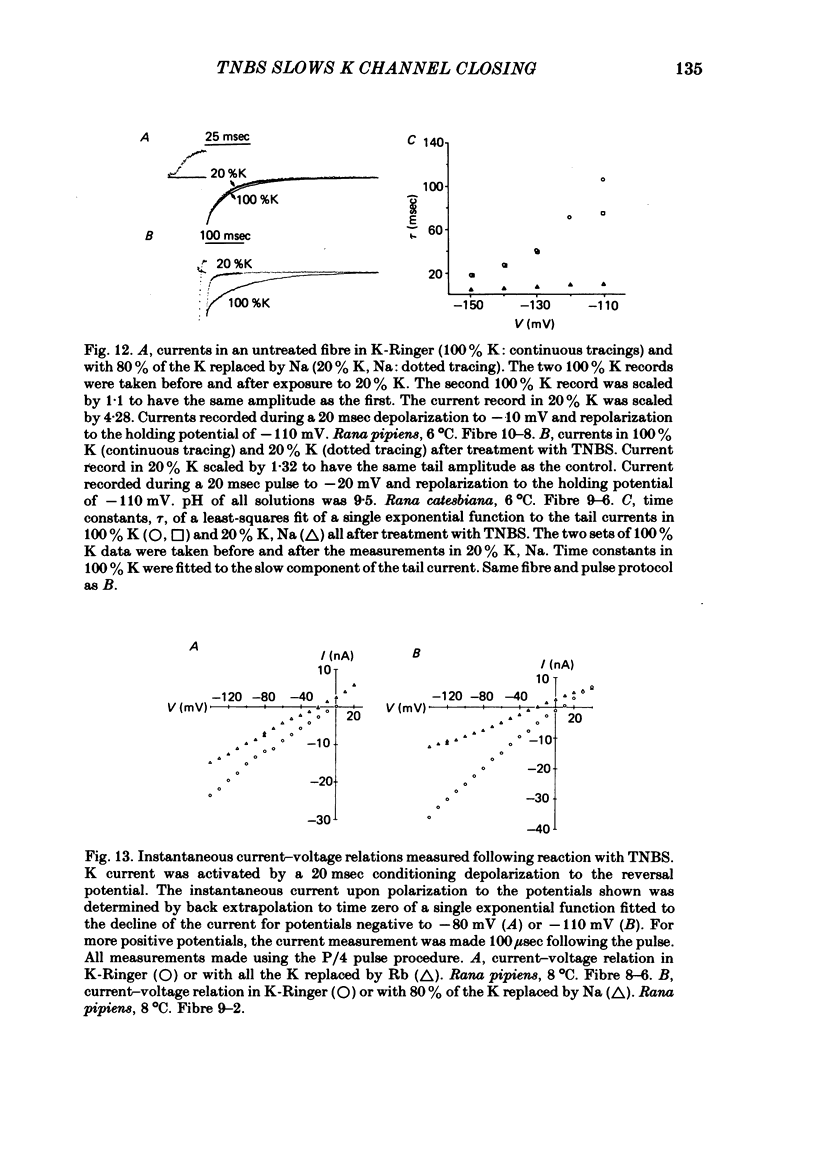
We investigated the actions of externally applied trinitrobenzene sulphonic acid (TNBS) on the K currents of voltage-clamped frog myelinated nerve fibres. TNBS treatment irreversibly slowed the tail currents for K channel closing up to 10-fold. Time constants for the tail currents appear shifted 60-80 mV in the hyperpolarizing direction following TNBS treatment. The time course of K channel opening was unaffected for depolarizing pulses to potentials positive to -20 mV. For smaller pulses to potentials between -80 and -30 mV the activation time course was slower following TNBS treatment. TNBS had little or no effect on the steady-state conductance-voltage relation of K channels determined from tail current amplitude. The instantaneous current-voltage relation for K channels and potency of block by external TEA were unaffected by TNBS. K tail currents showed fast and slow components both before and after TNBS treatment. Reaction with TNBS caused the fast component to decline in amplitude and the slow component to increase both in magnitude and time constant. The rate of reaction increased with increasing pH. Full expression of altered K channel kinetics depends upon the ionic composition of the external solution. Tail currents were up to 10-fold slower when measured in 117.5 mM-K than when measured with 80% of the external K replaced by Na. The differential effects of TNBS on K channel closing and opening were modelled in a three-state kinetic scheme, with an increase in the energy barrier for closing an open channel following TNBS treatment.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Nonner W., Dwyer T. M., Hille B. Block of endplate channels by permeant cations in frog skeletal muscle. J Gen Physiol. 1981 Dec;78(6):593–615. doi: 10.1085/jgp.78.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arhem P. Effects of rubidium, caesium, strontium, barium and lanthanum on ionic currents in myelinated nerve fibres from Xenopus laevis. Acta Physiol Scand. 1980 Jan;108(1):7–16. doi: 10.1111/j.1748-1716.1980.tb06494.x. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T. B., Cahalan M. D. Sodium channel permeation in squid axons. I: Reversal potential experiments. J Physiol. 1980 Oct;307:217–242. doi: 10.1113/jphysiol.1980.sp013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Hall J. Alamethicin channels incorporated into frog node of ranvier: calcium-induced inactivation and membrane surface charges. J Gen Physiol. 1982 Mar;79(3):411–436. doi: 10.1085/jgp.79.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Pappone P. A. Chemical modification of sodium channel surface charges in frog skeletal muscle by trinitrobenzene sulphonic acid. J Physiol. 1981 Dec;321:127–139. doi: 10.1113/jphysiol.1981.sp013975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. The steady-state potassium conductance of the Ranvier node at various external K-concentrations. Pflugers Arch. 1977 Aug 29;370(2):185–194. doi: 10.1007/BF00581693. [DOI] [PubMed] [Google Scholar]
- Dubois J. M. Evidence for the existence of three types of potassium channels in the frog Ranvier node membrane. J Physiol. 1981 Sep;318:297–316. doi: 10.1113/jphysiol.1981.sp013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Simultaneous changes in the equilibrium potential and potassium conductance in voltage clamped Ranvier node in the frog. J Physiol. 1981 Sep;318:279–295. doi: 10.1113/jphysiol.1981.sp013864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. A method for recording resting and action potentials in the isolated myelinated nerve fibre of the frog. J Physiol. 1957 Mar 11;135(3):550–559. doi: 10.1113/jphysiol.1957.sp005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Potassium permeability in myelinated nerve fibres of Xenopus laevis. J Physiol. 1962 Jan;160:54–61. doi: 10.1113/jphysiol.1962.sp006834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Van Helden D. Effects of permeant monovalent cations on end-plate channels. J Physiol. 1979 Mar;288:509–528. [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Divalent cations and the activation kinetics of potassium channels in squid giant axons. J Gen Physiol. 1982 Jun;79(6):965–996. doi: 10.1085/jgp.79.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol. 1975 Nov;66(5):535–560. doi: 10.1085/jgp.66.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971 Dec;58(6):599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il'in V. I., Katina I. E., Lonskii A. V., Makovskii V. S., Polishchuk E. V. Dannye o sushchestvovanii dvukh nezavisimykh komponent kalievogo toka v membrane perekhvata Ranv'e liagushki Rana ridibunda. Dokl Akad Nauk SSSR. 1977;234(6):1467–1470. [PubMed] [Google Scholar]
- Krylov B. V., Makovsky V. S. Spike frequency adaptation in amphibian sensory fibres is probably due to slow K channels. Nature. 1978 Oct 12;275(5680):549–551. doi: 10.1038/275549a0. [DOI] [PubMed] [Google Scholar]
- Marchais D., Marty A. Interaction of permeant ions with channels activated by acetylcholine in Aplysia neurones. J Physiol. 1979 Dec;297(0):9–45. doi: 10.1113/jphysiol.1979.sp013025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means G. E., Congdon W. I., Bender M. L. Reactions of 2,4,6-trinitrobenzenesulfonate ion with amines and hydroxide ion. Biochemistry. 1972 Sep 12;11(19):3564–3571. doi: 10.1021/bi00769a011. [DOI] [PubMed] [Google Scholar]
- Shrager P. Ionic conductance changes in voltage clamped crayfish axons at low pH. J Gen Physiol. 1974 Dec;64(6):666–690. doi: 10.1085/jgp.64.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Jr, Armstrong C. M. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 1981 Jun 4;291(5814):427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Effects of epinephrine on the pacemaker potassium current of cardiac Purkinje fibers. J Gen Physiol. 1974 Sep;64(3):293–319. doi: 10.1085/jgp.64.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]


