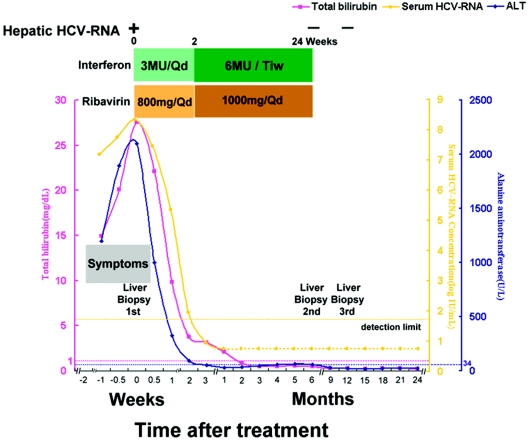
FIG. 1.
Clinical course and biochemical and molecular profiles of a patient with fulminant hepatitis C treated with alpha interferon and ribavirin combination therapy. Serum HCV RNA was determined by using a quantitative branched DNA assay (VERSANT HCV RNA 3.0 assay; Bayer Diagnostics) and a qualitative PCR assay (COBAS AMPLICOR Hepatitis C Virus Test, version 2.0; Roche). The levels of serum HCV RNA are presented as logarithmic transformations of original values; the horizontal dotted lines indicate the upper limits of the normal ranges. A dotted line indicates PCR negativity for serum HCV RNA, and a solid line indicates PCR positivity. Qd, daily; Tiw, three times a week; ALT, alanine aminotransferase.