Abstract
The incidence of measles virus (MV) infection has been significantly reduced in many nations through extensive vaccination; however, the virus still causes significant morbidity and mortality in developing countries. Measles outbreaks also occur in some developed countries that have failed to maintain high vaccine coverage rates. While vaccination is essential in preventing the spread of measles, case management would greatly benefit from the use of therapeutic agents to lower morbidity. Thus, the development of new therapeutic strategies is desirable. We previously reported the generation of a panel of small-molecule MV entry inhibitors. Here we show that our initial lead compound, although providing proof of concept for our approach, has a short half-life (<16 h) under physiological conditions. In order to combine potent antiviral activity with increased compound stability, a targeted library of candidate molecules designed on the structural basis of the first lead has been synthesized and tested against MV. We have identified an improved lead with low toxicity and high stability (half-life ≫ 16 h) that prevents viral entry and hence infection. This compound shows high MV specificity and strong activity (50% inhibitory concentration = 0.6 to 3.0 μM, depending on the MV genotype) against a panel of wild-type MV strains representative of viruses that are currently endemic in the field.
Paramyxoviruses are nonsegmented negative-stranded RNA viruses, most of which are highly contagious airborne pathogens that spread via the respiratory route. Members of this viral family constitute major human and animal pathogens such as measles virus (MV), human parainfluenza viruses (HPIV), mumps virus, rinderpest virus, and Newcastle disease virus (12). Despite the existence of an effective live-attenuated vaccine (6), MV remains a serious threat to human health globally, accounting for approximately 0.5 million deaths annually (1). While most of these cases occur in developing countries with limited access to vaccination, measles outbreaks still occur in some developed countries that have failed to maintain high vaccine coverage rates (4, 26). Recent outbreaks, in particular in the United Kingdom, have been attributed to declining herd immunity as a result of reduced vaccination coverage due to parental concerns about vaccination safety (8). Furthermore, vaccine-induced immunity is less robust than naturally acquired protection, which may, in fully vaccinated populations, result in a progressive loss of immunity in adults due to the absence of natural boosting through circulating virus (15, 16, 27). Taken together, these facts make desirable the development of novel therapeutics that could be produced cost-effectively and that could be used for the rapid control of local outbreaks and improved case management to limit severe outcomes of infection.
MV infects target cells through pH-independent fusion either of the viral envelope with the plasma membrane of target cells or of the plasma membrane of an infected cell with the plasma membrane of neighboring uninfected cells (11, 12). This is initiated by interaction of the hemagglutinin (H) envelope glycoprotein with its cellular receptor, either the regulator of complement activation CD46 or signaling lymphocyte activation molecule (SLAM/CD150w). While the MV vaccine strains of the Edmonston lineage efficiently use CD46 as their cellular receptor (3, 17), most wild-type strains of MV are dependent on SLAM for efficient entry (19, 32, 33). Receptor binding is thought to trigger H to activate the fusion (F) envelope glycoprotein, which through a series of conformational changes mediates membrane merger, resulting in release of the viral genome into the target cell (11, 12).
Interfering with virus entry is a novel and attractive therapeutic strategy to control virus infection and spread, and proof of principle for the clinical benefit of this approach has most notably come from the safe and efficacious peptidic human immunodeficiency virus (HIV) inhibitor enfuvirtide (T-20) (31). Paramyxoviruses against which peptides possess considerable in vitro potency include HPIV type 2 (HPIV-2) and HPIV-3 (13, 37), MV (13), respiratory syncytial virus (13), Sendai virus (28), and Newcastle disease virus (38). While confirming the therapeutic benefit of entry inhibitors for the treatment of viral infections, T-20 has highlighted potential obstacles that complicate large-scale production of peptide-based antivirals. Large heptad repeat-derived peptides such as T-20 are often difficult to solubilize and purify, making manufacture highly costly. Furthermore, such peptides usually show poor absorption and bioavailability from the gastrointestinal tract, necessitating delivery through injection, and virus-derived peptides have the potential to be immunogenic in vivo and may induce adverse events in some cases.
Considering these obstacles, we aimed to explore the inhibitory potential of nonpeptidic small molecules against MV entry. Multiple routes of administration are conceivable for these drug-like molecules, and highly cost-effective production strategies can be easily achieved. Additional conceptual support for this approach comes from the previous identification of small molecules that interfere with respiratory syncytial virus entry in vitro (2) and in vivo (2, 35).
In previous work (21, 25), we have reported the structure-guided development of an MV entry inhibitor, N-(5-amino-2-hydroxy-phenyl)-2-phenyl-acetamide (AM-4), with a 50% inhibitory concentration (IC50) of 260 nM against the MV vaccine strain MV-Edmonston (MV-Edm). In the present study, we further characterize this compound, describe the development of a second improved therapeutic lead, and report the testing of this lead against a representative panel of wild-type MV isolates. Based on its in vitro inhibitory activity against these strains, this compound warrants further testing under in vivo conditions.
MATERIALS AND METHODS
Cell culture, transfection, and production of MV stocks.
All cell lines were maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin. Vero (African green monkey kidney epithelial) cells (ATCC CCL-81) are non-contact-inhibited primate tumor cells. Vero-CD150w cells were derived from Vero cells by stably transfecting them with a human CD150w-expressing plasmid. They were incubated in the additional presence of G-418 (Geneticin) at a concentration of 0.35 mg/ml to maintain selective pressure. Lipofectamine 2000 (Invitrogen) was used for transient-transfection experiments according to the manufacturer's instruction. To prepare virus stocks, cells were infected at a multiplicity of infection (MOI) of 0.01 PFU/cell and incubated at 37°C. Cells were scraped in OPTIMEM (Invitrogen), and virus was released by two freeze-thaw cycles. MV-Edm and HPIV-2 stocks were grown and titered by 50% tissue culture infective dose (TCID50) determination on Vero cells according to the Spearman-Karber method, using the formula log10 (TCID50/ml) = L + d (s -0.5) + log10 (1/v) as previously described (23), where L is the negative log10 of the most concentrated virus dilution tested at which all wells are positive, d is the log10 of the dilution factor, s is the sum of individual proportions (pi; each pi is the calculated proportion of an individual dilution, and v is the volume of inoculum [ml/well]). All wild-type MV strains were grown and titered on Vero-CD150w cells using the same method.
Compound synthesis.
AM-4 and 5-amino-2-benzylbenzoxazole (OX-1) were synthesized and analyzed as previously described (21). For synthesis of 4-nitro-2-phenylaceylamino-benzamide (AS-48), 4-nitroanthranilic acid (202 mg, 1.0 mmol) in neat thionyl chloride (10 ml) was heated at 60°C for 3 h under N2 and concentrated by rotary evaporation. Residual SOCl2 was removed by three sequential additions of CH2Cl2 followed by rotary evaporation. Concentrated aqueous NH3 (10 ml) was added, and the mixture was stirred overnight at room temperature. The precipitates formed were collected by filtration and washed with water three times. The aqueous filtrate was extracted with ethyl acetate (three times, 5 ml) and dried over Na2SO4, and solvent was removed. The crude filtrate and the solid from filtrate extraction were combined and purified by recrystallization (methanol) to afford 2-amino-4-nitro-benzylamide (49 mg, 27% yield). The latter was dissolved in tetrahydrofuran, combined with 1.2 eq pyridine, and treated with 1.1 eq phenyl acetylchloride at room temperature for 3 h. The reaction mixture was poured into aqueous ammonium chloride, extracted with ethyl acetate, dried over Na2SO4, and chromatographed on silica gel (hexane/ethyl acetate at 1:1) to give AS-48 as a light yellow solid (54 mg, 67% yield). 1H nuclear magnetic resonance (NMR) (600 MHz, CDCl3) data are as follows: δ 3.79 (2H, s), 7.33-7.41(5H, m), 7.62 (1H, d, J = 8.4 Hz), 7.88 (1H, dd, J = 2.4 Hz, 8.4 Hz), 9.55 (1H, d, J = 1.8 Hz), 11.08 (1H, s). 13C NMR (400 MHz, CDCl3, ppm) data are as follows: 170.93, 141.71, 130.25, 129.71, 128.77, 128.29, 117.74, 117.14, 46.52. Combustion was performed based on the elemental composition C15H13N3O4. The calculated values based on the formula areas are as follows: C, 60.20; H, 4.38; N, 14.04. Values found experimentally are as follows: C, 60.29; H, 4.50; N, 13.27.
All compounds were dissolved in dimethyl sulfoxide (DMSO). The highest final DMSO concentration used in experiments was 0.2% (vol/vol), at which no solvent-related effect on cell viability or the degree of membrane fusion could be detected. As a control, cells treated with DMSO at the highest concentration used in each particular assay were routinely included.
Compound stability assays.
Compound stability was evaluated chemically by monitoring the NMR spectrum in CDCl3 under atmospheric oxygen every several hours over a 48-hour period. While OX-1 and AS-48 retained both solution clarity and spectrum integrity, AM-4 slowly darkened with some precipitation and the spectrum gradually showed the presence of impurities. Stability under physiological conditions was assessed by preincubation of different compound concentrations in OPTIMEM at 37°C for different time periods, followed by mixing with MV-Edm corresponding to an MOI of 0.1 PFU/cell and transfer to 5 × 105 target cells in a six-well plate format. Yields of cell-associated viral particles were determined 36 h postinfection by TCID50 titration.
Compound screen, cytotoxicity, and dose-response curves.
To screen candidate compounds, 7,500 Vero-CD150w cells per well were infected in four replicates per compound concentration in a 96-well plate format with wild-type strain MVi/Kansas. USA/43.00 (MV-KS) at an MOI of 0.1 PFU/cell in the presence of compound ranging from 75 μM to 4.6875 μM in twofold dilutions. Subsequent to incubation at 37°C for 96 h, virus-induced cytopathicity was measured using a cell proliferation assay (Promega) and results were calculated according to the formula % proliferation = [(experimental − background)/(maximum − background)] × 100. For comparison, the first-hit compound OX-1 was included in all assays.
A standard nonradioactive cytotoxicity assay (Promega) was used to determine the cytotoxicity of the compounds. In a 96-well plate format, 12,000 cells per well were incubated in four replicates per concentration tested in the presence of compound at 37°C for 24 h. Reduction of MTT [3-(4,5-dimethylthiazol-2-yl]-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] as an indicator for cell viability was then measured. Values were calculated according to the formula provided by the manufacturer: % cytotoxicity = [(experimental − background)/(maximum − background)] × 100. Due to the extreme fusogenicity of MV, in the absence of fusion inhibition essentially all cells of a monolayer were recruited into a single large syncytium at approximately 40 h postinfection in the conditions used in the study. Because of loss of mechanical stability, these giant cells detach from the plate and cease to be metabolically active. All infected cells were routinely assessed microscopically prior to each assay, and microscopic observations were consistent with the quantitative results obtained.
To generate dose-response curves, 4 × 105 Vero-CD150w cells per well were infected in a six-well plate format with wild-type MV strains at an MOI of 0.1 PFU/cell in the presence of AS-48 in twofold dilutions (75 μM highest) and incubated in the presence of compound at 37°C. Thirty-six hours postinfection, cells were scraped in OPTIMEM, cell-associated virus was released by two freeze/thaw cycles, and virus titers in cleared cell lysates (subsequent to centrifugation at 5,000 × g, 5 min, 4°C) were determined by TCID50 titration. IC50 values reflect the compound concentration at which virus yields were half that of solvent-only-treated control samples.
Isolation of envelope glycoproteins from primary MV isolates.
Total RNA was prepared from Vero-CD150w cells infected with the wild-type isolate of interest using the RNeasy minikit (QIAGEN) and subjected to reverse transcription using Superscript II reverse transcriptase (Invitrogen) and random hexamer primers. Genome fragments containing the F and H genes were then further amplified using TaqHiFi DNA-polymerase (Invitrogen), followed by transfer into TOPO 2.1 vectors (Invitrogen) and further cloning into pCG expression plasmids containing the constitutive cytomegalovirus promoter.
Transient-inhibition assays.
To determine the ability of candidate compounds to inhibit cell-to-cell fusion, 6 × 105 cells per well were transfected with 4 μg plasmid DNA each encoding MV-KS H and F genes, and cells were transferred 4 h posttransfection to 96-well plates with twofold compound dilutions ranging from 75 μM to 4.6875 μM in four replicates each. Fusion activity was assessed microscopically 48 h posttransfection, and the extent of cytotoxicity was quantified using the cytotoxicity assay (Promega) described above.
Membrane protection assays.
For entry experiments, virus inocula were transferred to 5 × 105 Vero-CD150w cells per well in a six-well plate format at an MOI of 0.1 PFU/cell in the presence of 50 μM AS-48 and incubated at 37°C for 60 min. Extracellular virions were inactivated by a 2-minute acid treatment (40 mM sodium citrate, 10 mM potassium chloride, 135 mM sodium chloride, pH 3.0) at 25°C, followed by extensive washing of cells and incubation at 37°C for 30 h. Control cells were subjected to washing steps only, and yields of cell-associated viral particles were determined by TCID50 titration.
RESULTS
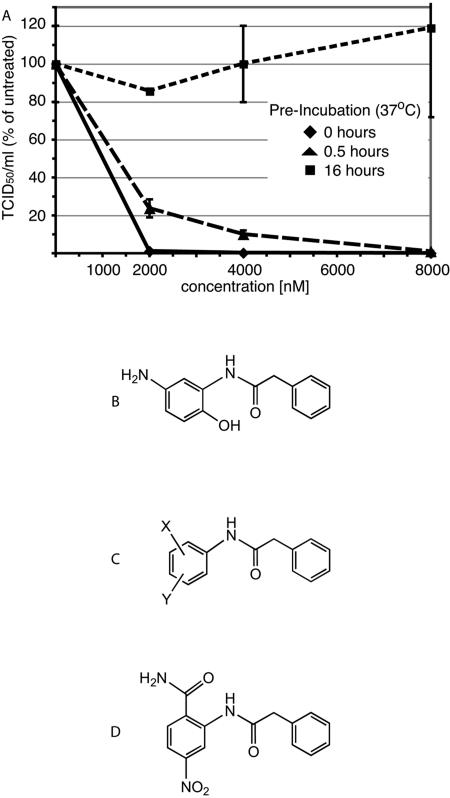
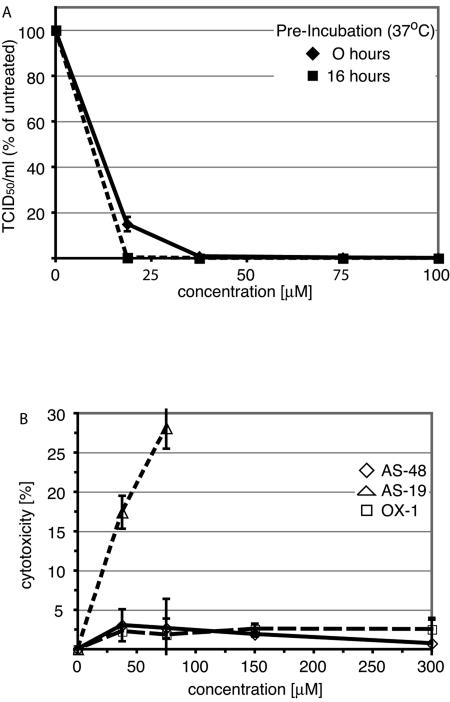
In previous work, we described an inhibitor of MV cell entry, AM-4 (21). Since this compound has promising antiviral activity against the MV-Edm strain (IC50 = 260 nM) we further investigated its potential as a therapeutic lead. In particular, a chemical half-life of at least 16 h under physiological conditions was considered a minimal requirement for compounds to warrant further testing. However, when AM-4 was subjected to in vitro stability testing at physiological pH and temperature, it was observed to be unstable. Preincubation for 16 h under these conditions fully ablated its antiviral activity (Fig. 1A). This observation was corroborated by high-performance liquid chromatography (HPLC) analysis of the compound subsequent to a 16-hour incubation period and by NMR sampling over a 48-hour period (data not shown).
FIG. 1.
Development of a stable therapeutic lead on the structural basis of compound AM-4. (A) AM-4 has a short half-life under physiological conditions. Serial dilutions of compound in growth medium were preincubated at 37°C as indicated, followed by transfer to cells and infection with MV. Virus yields were determined by TCID50 titration and are expressed in percentages of control cells infected in the presence of 0.01% DMSO. Values represent averages of two experiments performed in duplicate each. Standard deviations are shown. (B) Structure of AM-4. (C) Structure template of AM-4 used for structure-activity relationship development. (D) Structure of the improved lead compound AS-48.
Development of a stable lead inhibitor.
To develop an applicable MV inhibitor candidate, we therefore aimed to combine the antiviral properties of AM-4 with increased stability. Our approach was guided by speculation that the instability of AM-4 is due to its facile oxidation in air. Although we have not demonstrated the transformation, the para orientation of the OH and NH2 groups on the heterosubstituted aromatic ring (Fig. 1B) suggests ready oxidation to a quinone analog. Consequently, subsequent synthetic efforts were pursued with two principles in mind. First, substituent combinations on the benzamido ring of AM-4 analogs must be compatible with long-term exposure to air oxygen. Second, both substituent combination and orientation should provide steric and hydrogen bonding properties similar to AM-4 in terms of the previously developed MV homology model (21). These considerations led to the synthesis of a panel of 39 inhibitor candidates (to be described in detail elsewhere) by varying the substituent range of the structural backbone of AM-4, as depicted in Fig. 1C.
To evaluate compound activity against a clinically relevant, currently circulating MV strain (30), all candidate compounds were tested against MV-KS, a virus from genotype D5, which has been endemic in Thailand and Japan and was associated with imported cases in the United States and Europe (30). As a first screen, all compounds were assessed for their ability to suppress cytopathicity induced by MV-KS, as quantified by reduction of metabolic activity of cells using an MTT assay. Since cytopathicity due to extensive syncytium formation followed by giant cell detachment and death is a hallmark of MV infection in vitro, the inhibitory activity of the candidate compounds can be assessed in this assay by measuring increased cell survival and hence metabolic activity. When the antiviral activity of each candidate compound was compared with that of the stable first-hit compound OX-1 (21), the basis for all other compounds including AM-4, compound AS-48 (Fig. 1D) revealed the greatest increase in activity (7.8-fold increase compared to OX-1) and was therefore selected for further characterization.
Characterization of inhibitor candidate AS-48.
Structural predictions suggested that AS-48 would be air stable by comparison with AM-4 but still retain considerable activity. Indeed, preincubation of AS-48 at physiologic conditions for 16 h did not alter its inhibitory effect against MV (Fig. 2A), and HPLC analysis and NMR sampling over a 48-hour period (data not shows) likewise confirmed this assumption.
FIG. 2.
AS-48 is a chemically stable analog with low cytotoxicity. (A) AS-48 half-life exceeds 16 h under physiological conditions. Serial dilutions of compound in growth medium were preincubated at 37°C as indicated, followed by transfer to cells, infection with MV, and virus titration as described for Fig. 1A. Values represent averages of two experiments, each performed in duplicate. Standard deviations are shown. (B) AS-48 has low cytotoxicity, comparable to the first-hit compound OX-1. Cells were incubated in the presence of serial dilutions of compound for 24 to 30 h, followed by assessment of cytotoxicity. Values represent averages of four experiments and indicate percent cytotoxicity normalized for control cells treated with solvent (DMSO) only. Standard deviations are shown. For comparison, the first-hit compound OX-1 and a toxic candidate compound (AS-19) are included.
AS-48 was thus further characterized and its potential cytotoxicity assessed in a nonradioactive cytotoxicity assay performed in the presence of a range of different compound concentrations. We observed minimal cytotoxicity in compound-treated cells compared to solvent-only-treated control cells after 24 to 36 h of incubation in the presence of the compound (Fig. 2B). Cytotoxicity levels remained below 10% in the presence of compound concentrations as high as 300 μM. As controls, the first-hit compound OX-1, which showed no detectable cytotoxicity, and compound AS-19, which induces strong cytotoxicity at low concentrations, were also included in the assay. AS-48 did show some cytostatic effect at concentrations of 75 μM and higher, however, when cells were incubated in the presence of the compound for extended periods of time exceeding 4 days (data not shown).
Based on our previous characterization of the mechanism of antiviral activity of the first-hit compound OX-1, we postulated that the antiviral activity of AS-48 was also based on inhibition of the entry step of the virus life cycle. To test this hypothesis experimentally, we first examined whether AS-48 inhibits the cell-to-cell fusion activity induced by plasmid-encoded MV H and F glycoproteins. One would expect inhibition of membrane fusion if the compound indeed interferes with virus entry, while interference of AS-48 with downstream steps of the virus life cycle such as genome replication would suggest little inhibitory activity in a transient-fusion assay. We therefore isolated the genes encoding the H and F glycoproteins from MV-KS through reverse transcription-PCR of infected-cell lysates and transferred them into expression plasmids harboring the constitutive cytomegalovirus promoter. Vero cells stably expressing human CD150w showed extensive cell-to-cell fusion beginning at 12 h posttransfection when cotransfected with these plasmids (data not shown). But when cotransfected cells were mixed with a range of different AS-48 concentrations 4 h posttransfection and incubation was continued in the presence of different compound concentrations, cell-to-cell fusion was increasingly suppressed with rising concentration of AS-48. The extent of fusion inhibition was quantified based on the reduction of fusion-induced cytotoxicity (Fig. 3A). Dose-response curves are shown for AS-48-mediated inhibition of fusion induced by glycoproteins derived from MV-KS compared with those derived from the Edmonston vaccine strain cloned into otherwise identical expression plasmids (22).
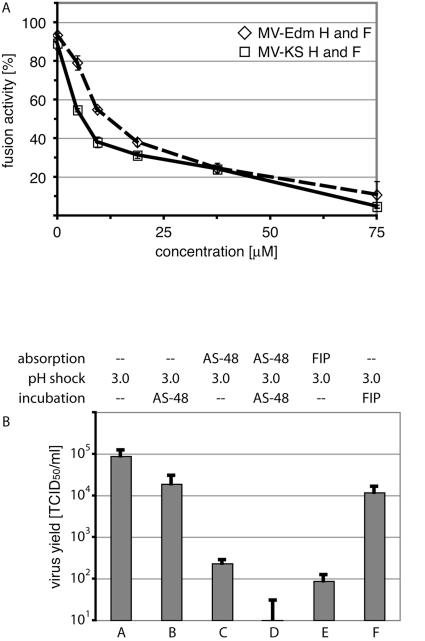
FIG. 3.
AS-48 prevents viral entry. (A) AS-48 inhibits cell-to-cell fusion induced by transiently expressed MV H and F glycoproteins derived from the MV-Edm and MV-KS strains, respectively. Values represent averages of four experiments and show quantification of cytopathicity as a consequence of membrane fusion. Standard deviations are given (B) Membrane protection assay to assess the entry status of viral particles. Particles were absorbed to target cells in the presence of either solvent alone (DMSO), AS-48, or FIP, followed by pH 3.0 treatment, two wash cycles, and incubation in the presence of DMSO, AS-48, or FIP as indicated. Virus yields were determined by TCID50 titration, and values represent averages of two experiments, each performed in duplicate. Standard deviations are given.
If AS-48 prevents membrane fusion and hence viral entry but not virus absorption to target cells or postentry steps of the viral life cycle, virions incubated with target cells in the presence of compound should be resistant to removal of the inoculum and washing but should remain sensitive to low-pH inactivation of extracellular MV particles. A brief pH 3.0 shock has been previously demonstrated to completely abolish the infectivity of many enveloped viruses, including MV, through inactivation of their fusogenic envelope glycoproteins (10, 21), while virions in a postentry state of their life cycle remain unaffected. Indeed, three consecutive wash steps after incubation of cells in the presence or absence of AS-48 did not result in a significant reduction in virus yields (Fig. 3B, samples A and B). When cells were exposed to low pH following incubation in the presence of compound, however, we found a strong reduction in virus yield when AS-48 was present (Fig. 3B, sample C). Furthermore, addition of the compound after particle absorption and low-pH treatment resulted in only a small reduction in virus yield, confirming that postentry steps of the viral life cycle are not targeted by AS-48 (Fig. 3B, sample B). The reduced extent of inhibition in sample C compared to control cells treated with AS-48 before and after the pH shock (Fig. 3B, sample D) is likely due to the escape of some particles from compound inhibition prior to low-pH treatment, while the reduction in virus yield in sample B compared to control infections in the absence of AS-48 is likely due to secondary infections being inhibited when cells are incubated in the presence of compound after low-pH treatment. These assumptions were confirmed by control samples in which AS-48 was replaced by fusion inhibitory peptide (FIP), a peptidic inhibitor of MV entry (18) that has been demonstrated previously to interfere with MV infection (Fig. 3B, samples E and F). Taken together, these findings support the conclusion that AS-48 acts at the level of MV entry.
Activity of AS-48 against relevant wild-type MV isolates.
To determine the target specificity of AS-48, we infected cells in the presence of a range of different compound concentrations with either MV-KS or HPIV-2, another member of the family Paramyxovirinae that is related to MV. Dose-response curves showing virus yields obtained after incubation in the presence of different compound concentrations reveal an IC50 for AS-48 against MV-KS of 0.6 μM, while yields of HPIV-2 are not affected even at the highest compound concentrations tested (Fig. 4). Likewise, AS-48 did not prevent membrane fusion induced by HIV type 1 (HIV-1) (data not shown), a member of the lentivirus family. These findings thus suggest the high target specificity of AS-48 and furthermore argue against residual cytotoxicity contributing to its inhibitory activity, since compound-mediated cytotoxicity would act nonspecifically and hence reduce yields of other viruses such as HPIV-2.
FIG. 4.
AS-48 shows high target specificity. Dose-response curves of AS-48 for MV-KS and HPIV-2 are shown. Cells were infected with MV-KS or HPIV-2 in the presence of serial dilutions of AS-48 as indicated, and virus titers were determined by TCID50 titration 36 h postinfection. Average values of two experiments are shown.
An IC50 of 0.6 μM against MV-KS confirms that AS-48 is more than seven times as potent as the first-hit compound OX-1 and only about 2.5-fold less active than the unstable compound AM-4. To further assess its potency against a representative panel of primary MV strains, we generated dose-response curves for viruses representing a relevant subset of genotypes that are currently active worldwide (30) and calculated IC50 values for each isolate (Fig. 5).
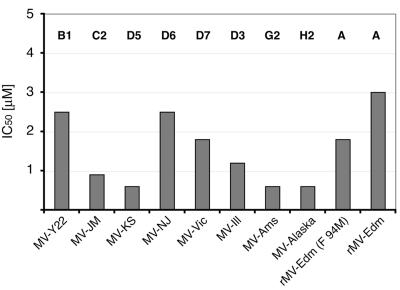
FIG. 5.
AS-48 is active against a representative panel of wild-type MV isolates. Dose-response curves were generated in duplicate experiments for a panel of currently endemic MV strains (MV-Y22, MVi/Yaounde.CAM/83 [B1]; MV-JM, MVi/Maryland.USA77 [C2]; MV-KS, D5; MV-NJ: MVi/New Jersey-1.USA [D6]; MV-Vic, MVi/Victoria.AUS/16.85 [D7]; MV-Ill, MVi/Illinois.USA/50.99 [D3]; MV-Ams, MVi-Amster.NET/49.97 [G2]; MV-Alaska, MVi/Alaska.USA/16.00 [H2]) representing genotypes B, C, D, G, and H. For comparison, the vaccine strain MV-Edm (genotype A) was included. Values indicate IC50 concentrations that were calculated on the basis of these inhibition curves.
AS-48 consistently demonstrated strong activity against all wild-type strains tested in this experiment, with IC50 values ranging from 0.6 μM to 2.5 μM depending on the strain. The lowest activity (IC50 = 3.0 μM) was observed for the vaccine strain MV-Edm (clade A), which has not been endemic for several decades. Consistent with our previous observations for the first-hit compound OX-1 (21), the lower sensitivity of the vaccine strain to inhibition by AS-48 may be at least partially due to a methionine-to-valine mutation at position 94 of the MV-Edm F protein. All wild-type strains analyzed so far contain a methionine residue at this position. Indeed, a recombinant MV-Edm (rMV-Edm F 94 M) in which this change has been reverted (20, 24) shows increased sensitivity to AS-48 (Fig. 5).
DISCUSSION
We describe the development of a potent and stable small-molecule inhibitor of MV entry, AS-48. This compound was designed on the basis of our previously reported MV entry inhibitor AM-4, which has strong antiviral activity but lacks stability. AS-48 demonstrates greatly improved stability under physiologic conditions, shows low cytotoxicity, and has potent antiviral activity, with an IC50 ranging from 0.6 to 2.5 μM against a panel of wild-type MV isolates representing currently circulating strains.
AM-4 is a masked hydroquinone with the NH2 group replacing the second OH on the aromatic ring. It is well known that several 1,2-, 1,3-, and 1,4-dihydroxybenzene analogs (pyrocatechol, resorcinol, and hydroquinone, respectively) are powerful antioxidants in fruits (34, 36), oils (29), and wine (7). Furthermore, the hydroquinone coenzyme Q (ubiquinol) is the membrane-sequestered small-molecule mediator of electron transport in the inner membrane of the mitochondria (5). Similarly, para-hydroxyanilines are also strong reducing agents that are readily air oxidized to quinones and related polymers (9). Consequently, the short half-life of AM-4 in air is understandable by analogy. We have thus attempted to introduce chemical stability, retain overall compound geometry, and present similar pharmacophoric elements compared to AM-4 in alternative structures. The nonplanar geometry of AM-4 (21) arises from the CH2 group serving as a fulcrum in the center of the molecule. It is retained in AS-48, as is the hydrophobic phenyl ring that we found to be critical for antiviral activity (21). The NH2 and OH substituents in the benzamido ring of AM-4 serve as proton donor and proton acceptor, respectively. The former is replaced by an amide, the latter by a nitro group in AS-48. As a result, the reducing capacity of the ring has been blocked. Indeed, the stability of AS-48 was confirmed experimentally both by HPLC and NMR, and by the fact that preincubation at physiologic conditions for 16 h caused negligible reduction of its antiviral activity.
Our data support the conclusion that AS-48 acts at the level of viral entry, as do its predecessors AM-4 and OX-1 (21). AS-48 inhibits cell-to-cell fusion induced by the H and F proteins of a currently prevalent genotype D5 strain, MV-KS (30), and does not interfere with postentry steps of the virus life cycle. Furthermore, our data suggest that AS-48, like AM-4 and OX-1, inhibits the membrane fusion step in the viral entry process, rather than interfering with the attachment of virus to target cells.
AS-48 demonstrated potent antiviral activity against a range of MV strains from genotypes that are currently circulating worldwide (30): B1, C2, D3, D5, D6, D7, G2, and H2. IC50 values ranging from 0.6 to 2.5 μM suggest that this compound may have potential as a therapeutic lead with activity against a broad range of MV strains. Additionally, AS-48 shows no activity against entry of the related paramyxovirus HPIV-2 or of the lentivirus HIV-1, demonstrating the target specificity of this compound for MV.
AS-48 and its predecessors AM-4 and OX-1 were all designed to dock into a microdomain in the MV F protein that we have previously described as important for the fusion process (21, 25). In previous work, we identified several mutations in this cavity and elsewhere in the F protein that conferred resistance to inhibition. One of these residues is amino acid 94, which is a valine in the MV-Edm vaccine strain and a methionine in all other strains analyzed thus far. MV-Edm shows a reduced susceptibility to inhibition with both AS-48 and OX-1, suggesting a role for this residue in determining sensitivity to inhibition by this family of inhibitors. Consistent with this observation, substituting valine for methionine in MV-Edm conferred increased sensitivity to AS-48 on a recombinant virus.
We reason that the development of small-molecule measles virus inhibitors that can be produced cost-effectively may be highly beneficial in complementing the existing measles vaccination program by rapidly controlling local outbreaks and augmenting management of severe cases of measles. Since MV infects through the respiratory route and our compounds or derivatives thereof could easily be aerosolized for oral administration, we furthermore consider a prophylactic application as an attractive future possibility. We predict that viral spread could be drastically reduced in a setting similar to the recent outbreaks reported in the United Kingdom if an effective inhibitor were made available prophylactically to nonimmunized individuals in direct contact with identified index cases. Indeed, computer simulation models have demonstrated that an influenza pandemic could be blocked at the onset if antiviral drugs were administered to susceptible contacts of index cases (14).
Based on its activity against a range of measles virus strains currently circulating worldwide, we hypothesize that AS-48 may have significant potential as a therapeutic lead inhibitor. The potent in vitro antiviral activity of AS-48 reported here supports further testing in vivo to assess the candidacy of AS-48 for clinical development.
Acknowledgments
We are grateful to A. N. Vzorov for testing of compound activity against HIV-1 and to L. K. White for critical reading of the manuscript.
This work was supported by a pilot grant from the Southeastern Center for Emerging Biological Threats (grant/cooperative agreement number U38/CCU423095 from CDC), a research grant from the American Lung Association, and Public Health Service grant 1R21AI056179-01A2 from NIH/NIAID (all to R.K.P.) and Public Health Service grant AI057157 to the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2005. Progress in reducing measles mortality—worldwide, 1999-2003. Morb. Mortal. Wkly. Rep. 54:200-203. [PubMed] [Google Scholar]
- 2.Cianci, C., D. R. Langley, D. D. Dischino, Y. Sun, K. L. Yu, A. Stanley, J. Roach, Z. Li, R. Dalterio, R. Colonno, N. A. Meanwell, and M. Krystal. 2004. Targeting a binding pocket within the trimer-of-hairpins: small-molecule inhibition of viral fusion. Proc. Natl. Acad. Sci. USA 101:15046-15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 4.Gans, H. A., A. M. Arvin, J. Galinus, L. Logan, R. DeHovitz, and Y. Maldonado. 1998. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA 280:527-532. [DOI] [PubMed] [Google Scholar]
- 5.Garrett, R. H., and C. M. Grisham. 2005. Biochemistry, 3rd ed. Thomson Learning, Inc., Stamford, Conn.
- 6.Hilleman, M. R. 2001. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine 20:651-665. [DOI] [PubMed] [Google Scholar]
- 7.Jang, M., L. Cai, G. O. Udeani, K. V. Slowing, C. F. Thomas, C. W. Beecher, H. H. Fong, N. R. Farnsworth, A. D. Kinghorn, R. G. Mehta, R. C. Moon, and J. M. Pezzuto. 1997. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218-220. [DOI] [PubMed] [Google Scholar]
- 8.Jansen, V. A., N. Stollenwerk, H. J. Jensen, M. E. Ramsay, W. J. Edmunds, and C. J. Rhodes. 2003. Measles outbreaks in a population with declining vaccine uptake. Science 301:804. [DOI] [PubMed] [Google Scholar]
- 9.Kaya, I. 2004. Synthesis, characterization, and optimum reaction conditions of oligo-benzylidene-3′-hydroxyaniline. Int. J. Polymer Anal. Charact. 9:137-151. [Google Scholar]
- 10.Kizhatil, K., and L. M. Albritton. 1997. Requirements for different components of the host cell cytoskeleton distinguish ecotropic murine leukemia virus entry via endocytosis from entry via surface fusion. J. Virol. 71:7145-7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb, R. A. 1993. Paramyxovirus fusion: a hypothesis for changes. Virology 197:1-11. [DOI] [PubMed] [Google Scholar]
- 12.Lamb, R. A., and D. Kolakosky. 1996. Paramyxoviridae: the viruses and their replication, p. 1305-1340. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 13.Lambert, D. M., S. Barney, A. L. Lambert, K. Guthrie, R. Medinas, D. E. Davis, T. Bucy, J. Erickson, G. Merutka, and S. R. Petteway, Jr. 1996. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc. Natl. Acad. Sci. USA 93:2186-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longini, I. M., Jr., M. E. Halloran, A. Nizam, and Y. Yang. 2004. Containing pandemic influenza with antiviral agents. Am. J. Epidemiol. 159:623-633. [DOI] [PubMed] [Google Scholar]
- 15.Mossong, J., D. J. Nokes, W. J. Edmunds, M. J. Cox, S. Ratnam, and C. P. Muller. 1999. Modeling the impact of subclinical measles transmission in vaccinated populations with waning immunity. Am. J. Epidemiol. 150:1238-1249. [DOI] [PubMed] [Google Scholar]
- 16.Mossong, J., C. J. O'Callaghan, and S. Ratnam. 2000. Modelling antibody response to measles vaccine and subsequent waning of immunity in a low exposure population. Vaccine 19:523-529. [DOI] [PubMed] [Google Scholar]
- 17.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norrby, E. 1971. The effect of a carbobenzoxy tripeptide on the biological activities of measles virus. Virology 44:599-608. [DOI] [PubMed] [Google Scholar]
- 19.Oldstone, M. B., D. Homann, H. Lewicki, and D. Stevenson. 2002. One, two, or three step: measles virus receptor dance. Virology 299:162-163. [DOI] [PubMed] [Google Scholar]
- 20.Plemper, R. K., and R. W. Compans. 2003. Mutations in the putative HR-C region of the measles virus F2 glycoprotein modulate syncytium formation. J. Virol. 77:4181-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plemper, R. K., K. J. Erlandson, A. S. Lakdawala, A. Sun, A. Prussia, J. Boonsombat, E. Aki-Sener, I. Yalcin, I. Yildiz, O. Temiz-Arpaci, B. Tekiner, D. C. Liotta, J. P. Snyder, and R. W. Compans. 2004. A target site for template-based design of measles virus entry inhibitors. Proc. Natl. Acad. Sci. USA 101:5628-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plemper, R. K., A. L. Hammond, and R. Cattaneo. 2000. Characterization of a region of the measles virus hemagglutinin sufficient for its dimerization. J. Virol. 74:6485-6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plemper, R. K., A. L. Hammond, and R. Cattaneo. 2001. Measles virus envelope glycoproteins hetero-oligomerize in the endoplasmic reticulum. J. Biol. Chem. 276:44239-44246. [DOI] [PubMed] [Google Scholar]
- 24.Plemper, R. K., A. L. Hammond, D. Gerlier, A. K. Fielding, and R. Cattaneo. 2002. Strength of envelope protein interaction modulates cytopathicity of measles virus. J. Virol. 76:5051-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plemper, R. K., A. S. Lakdawala, K. M. Gernert, J. P. Snyder, and R. W. Compans. 2003. Structural features of paramyxovirus F protein required for fusion initiation. Biochemistry 42:6645-6655. [DOI] [PubMed] [Google Scholar]
- 26.Polack, F. P., S. H. Lee, S. Permar, E. Manyara, H. G. Nousari, Y. Jeng, F. Mustafa, A. Valsamakis, R. J. Adams, H. L. Robinson, and D. E. Griffin. 2000. Successful DNA immunization against measles: neutralizing antibody against either the hemagglutinin or fusion glycoprotein protects rhesus macaques without evidence of atypical measles. Nat. Med. 6:776-781. [DOI] [PubMed] [Google Scholar]
- 27.Putz, M. M., F. B. Bouche, R. L. de Swart, and C. P. Muller. 2003. Experimental vaccines against measles in a world of changing epidemiology. Int. J. Parasitol. 33:525-545. [DOI] [PubMed] [Google Scholar]
- 28.Rapaport, D., M. Ovadia, and Y. Shai. 1995. A synthetic peptide corresponding to a conserved heptad repeat domain is a potent inhibitor of Sendai virus-cell fusion: an emerging similarity with functional domains of other viruses. EMBO J. 14:5524-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche, M., C. Dufour, N. Mora, and O. Dangles. 2005. Antioxidant activity of olive phenols: mechanistic investigation and characterization of oxidation products by mass spectrometry. Org. Biomol. Chem. 3:423-430. [DOI] [PubMed] [Google Scholar]
- 30.Rota, P. A., and W. J. Bellini. 2003. Update on the global distribution of genotypes of wild type measles viruses. J. Infect. Dis. 187(Suppl. 1):S270-S276. [DOI] [PubMed] [Google Scholar]
- 31.Starr-Spires, L. D., and R. G. Collman. 2002. HIV-1 entry and entry inhibitors as therapeutic agents. Clin. Lab. Med. 22:681-701. [DOI] [PubMed] [Google Scholar]
- 32.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 33.Tatsuo, H., N. Ono, and Y. Yanagi. 2001. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 75:5842-5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinson, J. A., X. Su, L. Zubik, and P. Bose. 2001. Phenol antioxidant quantity and quality in foods: fruits. J. Agric. Food Chem. 49:5315-5321. [DOI] [PubMed] [Google Scholar]
- 35.Weiss, W. J., T. Murphy, M. E. Lynch, J. Frye, A. Buklan, B. Gray, E. Lenoy, S. Mitelman, J. O'Connell, S. Quartuccio, and C. Huntley. 2003. Inhalation efficacy of RFI-641 in an African green monkey model of RSV infection. J. Med. Primatol. 32:82-88. [DOI] [PubMed] [Google Scholar]
- 36.Yan, X., B. T. Murphy, G. B. Hammond, J. A. Vinson, and C. C. Neto. 2002. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 50:5844-5849. [DOI] [PubMed] [Google Scholar]
- 37.Yao, Q., and R. W. Compans. 1996. Peptides corresponding to the heptad repeat sequence of human parainfluenza virus fusion protein are potent inhibitors of virus infection. Virology 223:103-112. [DOI] [PubMed] [Google Scholar]
- 38.Young, J. K., R. P. Hicks, G. E. Wright, and T. G. Morrison. 1997. Analysis of a peptide inhibitor of paramyxovirus (NDV) fusion using biological assays, NMR, and molecular modeling. Virology 238:291-304. [DOI] [PubMed] [Google Scholar]