Abstract
A large amount of knowledge has been acquired since the original descriptions of Lyme borreliosis (LB) and of its causative agent, Borrelia burgdorferi sensu stricto. The complexity of the organism and the variations in the clinical manifestations of LB caused by the different B. burgdorferi sensu lato species were not then anticipated. Considerable improvement has been achieved in detection of B. burgdorferi sensu lato by culture, particularly of blood specimens during early stages of disease. Culturing plasma and increasing the volume of material cultured have accomplished this. Further improvements might be obtained if molecular methods are used for detection of growth in culture and if culture methods are automated. Unfortunately, culture is insensitive in extracutaneous manifestations of LB. PCR and culture have high sensitivity on skin samples of patients with EM whose diagnosis is based mostly on clinical recognition of the lesion. PCR on material obtained from extracutaneous sites is in general of low sensitivity, with the exception of synovial fluid. PCR on synovial fluid has shown a sensitivity of up to >90% (when using four different primer sets) in patients with untreated or partially treated Lyme arthritis, making it a helpful confirmatory test in these patients. Currently, the best use of PCR is for confirmation of the clinical diagnosis of suspected Lyme arthritis in patients who are IgG immunoblot positive. PCR should not be used as the sole laboratory modality to support a clinical diagnosis of extracutaneous LB. PCR positivity in seronegative patients suspected of having late manifestations of LB most likely represents a false-positive result. Because of difficulties in direct methods of detection, laboratory tests currently in use are mainly those detecting antibodies to B. burgdorferi sensu lato. Tests used to detect antibodies to B. burgdorferi sensu lato have evolved from the initial formats as more knowledge on the immunodominant antigens has been collected. The recommendation for two-tier testing was an attempt to standardize testing and improve specificity in the United States. First-tier assays using whole-cell sonicates of B. burgdorferi sensu lato need to be standardized in terms of antigen composition and detection threshold of specific immunoglobulin classes. The search for improved serologic tests has stimulated the development of recombinant protein antigens and the synthesis of specific peptides from immunodominant antigens. The use of these materials alone or in combination as the source of antigen in a single-tier immunoassay may someday replace the currently recommended two-tier testing strategy. Evaluation of these assays is currently being done, and there is evidence that certain of these antigens may be broadly cross-reactive with the B. burgdorferi sensu lato species causing LB in Europe.
INTRODUCTION
Lyme borreliosis (LB), or Lyme disease, which is transmitted by ticks of the Ixodes ricinus complex, was described as a new entity in the United States in the late 1970s (318, 319, 324, 325). Many of its individual manifestations had been documented many decades earlier in Europe (355). The etiologic agent, Borrelia burgdorferi, was recovered first in 1982 from the vector tick Ixodes dammini (now I. scapularis) (41) and subsequently from skin biopsy, cerebrospinal fluid (CSF), or blood specimens of patients with LB in the United States (22, 323) and Europe (3, 14, 268). In the United States, the Centers for Disease Control and Prevention (CDC) initiated surveillance for LB in 1982, and the Council of State and Territorial Epidemiologists adopted a resolution making LB a nationally notifiable disease in 1990. LB is the most common vector-borne disease in North America and represents a major public health challenge for the medical community. Since 1982, more than 200,000 LB cases in the United States have been reported to the CDC, with about 17,000 cases reported yearly between 1998 and 2001 (54). In 2002 the number of cases of LB in the United States increased to 23,763, with a national incidence of 8.2 cases per 100,000 population. Approximately 95% of the cases occurred in 12 states located in the northeastern, mid-Atlantic, and north central regions (54); these states were Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Wisconsin. LB is widely distributed in European countries and also occurs in far eastern Russia and in some Asian countries (128, 288, 351).
During the past 20 years, notable advances have been made in understanding the etiologic agent, B. burgdorferi, and the illness that it causes. Many excellent reviews have been published detailing progress in expanding the knowledge base on the microbiology of B. burgdorferi (20, 29, 50, 283, 307, 351, 353) and on the ecology and epidemiology (12, 40, 247, 297, 299), pathogenesis (127, 225, 321, 335, 357), clinical aspects (219, 253, 313-316, 372), and laboratory diagnosis (18, 39, 291, 360, 362, 365) of LB. It has been estimated that more than 2.7 million serum samples are tested each year for the presence of B. burgdorferi-specific antibodies in the United States alone (339). To meet the demand for laboratory-based diagnosis, various new tests for direct detection of the etiologic agent, or for detection of specific antibodies by using whole-cell lysates, recombinant antigens, or peptide antigens in enzyme immunoassays (EIA), have been introduced into the clinical laboratory. This review attempts to provide a comprehensive assessment of the development and application of currently available tests for the laboratory diagnosis of LB. Future directions for improvement of established tests and for development of new approaches are also discussed.
CHARACTERISTICS OF B. BURGDORFERI
B. burgdorferi is a helically shaped bacterium with multiple endoflagella. The cells, configured with 3 to 10 loose coils, are 10 to 30 μm in length and 0.2 to 0.5 μm in width (20). This spirochete possesses several morphological, structural, ecologic, and genomic features that are distinctive among prokaryotes.
Cultured B. burgdorferi organisms are motile and swim in freshly prepared slides. Live organisms can be visualized by dark-field or phase-contrast microscopy. They can also be recognized by light microscopy after staining with silver stains or by fluorescent microscopic methods. The ultrastructure of B. burgdorferi is comprised of an outer slime surface layer (S-layer), a trilaminar outer membrane surrounding the periplasmic space that usually contains 7 to 11 periplasmic flagella and an innermost compartment, the protoplasmic cylinder (120). Detailed information on the cell structure and biology of B. burgdorferi is found in published reviews (20, 353).
B. burgdorferi is the first spirochete whose complete genome was sequenced (98). The genome size of the type strain B. burgdorferi sensu stricto B31 is 1,521,419 bp. This genome consists of a linear chromosome of 910,725 bp, with a G+C content of 28.6%, and 21 plasmids (9 circular and 12 linear) which have a combined size of 610,694 bp (52, 98). Comparative analysis of the genome of the recently sequenced Borrelia garinii strain PBi with that of B. burgdorferi B31 reveals that most of the chromosome is conserved (92.7% identity with regard to both DNA and amino acids) in the two species. The chromosome and two linear plasmids (lp54 and cp26), which carry approximately 860 genes, seem to belong to the basic genome inventory of the Lyme Borrelia species (105). Not all strains of B. burgdorferi have the complete complement of plasmids, and thus the cumulative genome size may vary among different B. burgdorferi isolates.
Genome analysis has revealed that B. burgdorferi possesses certain genetic structures that are uncommon among prokaryotes (98). These include (i) a linear chromosome and multiple linear and circular plasmids in a single bacterium; (ii) a unique organization of the rRNA gene cluster, consisting of a single 16S rRNA gene (rrs) and tandemly repeated 23S (rrl) and 5S (rrf) rRNA genes; (iii) over 150 lipoprotein-encoding genes, which account for 4.9% of the chromosomal genes and 14.5% of the plasmid genes, significantly higher than that of any other bacterial genome sequenced to date; (iv) a substantial fraction of plasmid DNA that appears to be in a state of evolutionary decay; (v) evidence for numerous, potentially recent DNA rearrangements among the plasmid genes; and (vi) a lack of recognized genes that encode enzymes required for synthesis of amino acids, fatty acids, enzyme cofactors, and nucleotides. B. burgdorferi also lacks genes coding for tricarboxylic acid cycle enzymes or for compounds involved in electron transport, findings which, taken together with the preceding, indicate the parasitic nature of this microorganism (52, 98, 353).
B. burgdorferi Genospecies
Eleven Borrelia species within the B. burgdorferi sensu lato complex have been described worldwide (16, 49, 99, 149, 168, 194, 264, 350). Of these, three species (B. burgdorferi sensu stricto, Borrelia andersonii, and Borrelia bissettii) have been identified in North America, five species (B. burgdorferi sensu stricto, B. garinii, Borrelia afzelii, Borrelia valaisiana, and Borrelia lusitaniae) have been recognized in Europe, and seven species (B. garinii, B. afzelii, B. valaisiana, Borrelia japonica, Borrelia tanukii, Borrelia turdi, and Borrelia sinica) have been identified in Asian countries (e.g., China, Japan, or Korea). Identification and differentiation of B. burgdorferi sensu lato species can be achieved by using several molecular approaches, which are reviewed elsewhere (351).
At least three B. burgdorferi sensu lato species, i.e., B. burgdorferi sensu stricto, B. garinii, and B. afzelii, are pathogenic to humans in Europe (342, 351). In contrast, B. burgdorferi sensu stricto is the sole species known to cause human infection in the United States (195). However, several subtypes of B. burgdorferi sensu stricto have been identified (175, 176), and an association between specific subtypes of B. burgdorferi sensu stricto and hematogenous dissemination and invasion in patients (304, 370) and experimentally infected animals (348, 349) has been reported.
Of the seven B. burgdorferi sensu lato species identified in Asia, only B. garinii and B. afzelii have been confirmed definitively to be pathogenic in humans. Although studies with both patients and laboratory animals have indicated the potential for B. bissettii, B. valaisiana, or B. lusitaniae to cause clinical disease (65, 81, 106, 257, 334), the pathogenicity of these Borrelia species in humans is not well established.
The genetic relatedness of B. burgdorferi sensu lato isolates has been compared at the species, subspecies, or single gene level by various molecular approaches. Earlier analyses of B. burgdorferi sensu lato isolates representing different species suggested that these species had highly conserved chromosomal gene orders (51, 232) with linear plasmid profiles similar to that described for the B. burgdorferi sensu stricto type strain B31 (243). Recent studies, however, have documented genetic heterogeneity among B. burgdorferi sensu lato isolates in the United States (173, 195) and in Europe (263, 288, 352). A large number of DNA sequences of B. burgdorferi sensu lato genes are now available in the GenBank database from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/entrez/). Examples include fla, vlsE, bmpA, and dbpA and genes encoding B. burgdorferi sensu lato 16S rRNA and outer surface proteins A and C.
It is well known that B. burgdorferi sensu lato expresses different surface proteins in adaptation to various microenvironments (79, 95, 299). For example, the spirochete expresses OspA but not OspC when residing in the midguts of unfed ticks. However, during a blood meal by the tick, some spirochetes stop expressing OspA and instead express OspC (230, 298). Certain B. burgdorferi sensu lato genes either are expressed only in a mammalian host or have significantly up-regulated expression in that environment; such gene products include VlsE (71, 230), DbpA (71, 129), BBK32 (96), Erp (202), and Mlp (376) proteins. Recently, whole-genome microarrays were employed to analyze gene expression of B. burgdorferi sensu stricto grown under conditions analogous to those found in unfed ticks, fed ticks, and mammalian hosts (32, 220, 231, 278). Gene expression analysis of B. burgdorferi B31 grown at 23°C and 35°C, to simulate temperatures found in tick vectors and mammalian hosts, respectively, demonstrated that a total of 215 open reading frames were differentially expressed at the two temperatures. Strikingly, 136 (63%) of the differentially expressed genes were plasmid carried, which highlights the potential importance of plasmid-carried genes in the adjustment of B. burgdorferi sensu lato to diverse environmental conditions (231).
Genetic diversity and differential expression of B. burgdorferi sensu lato genes in patients have important implications for development of molecular assays and serologic tests in the laboratory diagnosis of LB. As discussed in the following sections, the choice of PCR primers targeting different segments of the B. burgdorferi sensu lato genome, as well as the selection of particular antigens for serologic assays, may affect the sensitivities and specificities of these diagnostic assays (252, 291).
LYME BORRELIOSIS: DISEASE SPECTRUM
Infection with B. burgdorferi sensu lato can result in dermatological, neurological, cardiac, and musculoskeletal disorders. The basic clinical spectra of the disease are similar worldwide, although differences in clinical manifestations between LB occurring in Europe and North America are well documented (219, 316, 351). Such differences are attributed to differences in B. burgdorferi sensu lato species causing LB on the two continents. Furthermore, differences in clinical presentations exist between regions of Europe, presumably due to differences in the rates of occurrence of infection caused by distinct B. burgdorferi sensu lato species (128, 288, 342).
Patients with B. burgdorferi sensu lato infection may experience one or more clinical syndromes of early or late LB. Usually, early infection consists of localized erythema migrans (EM), which may be followed within days or weeks by clinical evidence of disseminated infection that may affect the skin, nervous system, heart, or joints and subsequently, within months, by late infection (219, 314, 316, 318, 373). Arthritis appears to be more frequent in North American patients (326, 332), whereas lymphocytoma, acrodermatitis chronica atrophicans (ACA), and encephalomyelitis have been seen primarily in Europe (313).
EM is the characteristic sign of early infection with B. burgdorferi sensu lato and the clinical hallmark of LB. In recent series it is recognized in at least 80% of patients with objective clinical evidence of B. burgdorferi sensu lato infection who meet the CDC surveillance definition of LB (327). The rash begins at the site of the tick bite as a red macule or papule, rapidly enlarges, and sometimes develops central clearing. The clinical diagnosis of early LB with EM relies on recognition of the characteristic appearance of a skin lesion of at least 5 cm in diameter. At this stage, patients may either be asymptomatic or, more commonly in the United States, experience flu-like symptoms, such as headache, myalgia, arthralgias, or fever (309, 332). The presence of constitutional signs and symptoms in a patient with EM has been considered evidence of dissemination by some investigators, but this is not evidence based (192). Instead, we will refer to this clinical presentation as symptomatic EM later in this review.
Hematogenous dissemination of B. burgdorferi sensu lato to the nervous system, joints, heart, or other skin areas, and occasionally to other organs, may give rise to a wide spectrum of clinical manifestations of what is called early LB. Usually, patients with objective evidence of dissemination experience one or more of the following syndromes: multiple EM lesions, atrioventricular conduction defects, myopericarditis, arthritis, facial palsy, meningitis, and meningoradiculoneuritis (Bannwarth's syndrome) (238, 333, 343).
Late LB may develop among some untreated patients months to a few years after tick-transmitted infection. The major manifestations of late LB include arthritis, late neuroborreliosis (peripheral neuropathy or encephalomyelitis), and ACA. Lyme arthritis begins as intermittent attacks of mono- or pauciarticular arthritis, especially of large joints. In up to 10% of patients, arthritis may persist for months or a few years despite treatment with antimicrobials. Treatment-resistant arthritis is more frequently seen in patients with the certain HLA DRB alleles (112, 145). It has been suggested that autoimmunity plays a role in this clinical entity (320, 338).
While Lyme arthritis is the most common late manifestation of LB in North America, ACA appears to be the most common manifestation of late LB in Europe. As mentioned earlier, these differences are likely due to the different species causing LB in the two continents (219, 316, 351).
More details on the clinical spectra of LB are found in recent reviews (219, 313, 314, 316, 372).
LABORATORY DIAGNOSIS
Direct Detection of B. burgdorferi
A variety of laboratory techniques have been developed for direct detection of B. burgdorferi sensu lato These assays provide evidence for the presence of intact spirochetes or spirochete components such as DNA or protein in tick vectors, reservoir hosts, or patients.
Four different approaches have been used in the clinical laboratory: microscope-based assays, detection of B. burgdorferi-specific proteins or nucleic acids, and culture. Of these, culture of B. burgdorferi sensu lato undoubtedly offers the best confirmation of active infection and has been increasingly used as a diagnostic modality by many researchers on both sides of the Atlantic. The availability of cultured organisms has also allowed investigation of the structural, molecular, antigenic, and pathogenetic properties of the different B. burgdorferi sensu lato species.
Direct microscopic detection of B. burgdorferi sensu lato has limited clinical utility in laboratory confirmation of LB due to the sparseness of organisms in clinical samples (24, 27, 75-77, 125, 139, 153, 156, 190, 212, 221, 310). Antigen detection assays (aside from PCR) also suffer from the same limitations as microscopic detection. Although antigen capture tests have been used to detect B. burgdorferi sensu lato antigens in CSF of patients with neuroborreliosis (67, 69) and in urine samples from patients with suspected LB (83, 131, 155), their reliability is poor or at best questionable (155). Our review of direct methods of detection of B. burgdorferi sensu lato in clinical samples will focus on culture and molecular methods.
Culture of B. burgdorferi sensu lato. (i) Culture techniques.
The liquid media currently used to grow B. burgdorferi sensu lato were derived from the original Kelly medium (152) through various modifications made over time (17, 19, 267, 328, 329). Current versions of this medium (Barbour-Stoenner-Kelly II medium [BSK II] [17], BSK-H [262], and Kelly medium Preac-Mursic [MKP] [267]) are better able to support growth of B. burgdorferi sensu lato in terms of recovery from low inocula, shorter generation times of the spirochete, and maximal concentration of spirochetes in culture (∼108 to 109/ml) (Table 1). Key ingredients of BSK II include CMRL-1066, which is a standard medium used for growing various types of mammalian cells; bovine serum albumin fraction V, which serves as a rich source of protein and to stabilize the pH; N-acetylglucosamine, a precursor for bacterial cell wall biosynthesis; rabbit serum; citrate; pyruvate; and many others.
TABLE 1.
Selected features of various modifications of Kelly medium
| Name | Description (reference[s]) |
|---|---|
| Stoenner modification | Addition of Yeastolate and CMRL 1066, without glutamine and without sodium bicarbonate (329) |
| Barbour modification (BSK II) | Use of CMRL 1066 without glutamine; Yeastolate; neopeptone as the peptone preparation; and HEPES as a buffer (17, 19) |
| Preac-Mursic modification (MKPa) | Removal of Yeastolate; different proportions of certain ingredients (267) |
| BSK-H | Deletion of gelatin; different proportions of certain ingredients (262) |
Also referred to as the Pettenkofer modification (256).
The growth-promoting capability of BSK II and related media depends upon the careful selection of certain key components that may be highly variable in composition depending on their source (262), particularly the specific preparations of bovine serum albumin (43) and rabbit serum (262). For example, a minority of preparations of rabbit serum contain antispirochetal antibodies, and inclusion of such preparations in the medium will reduce or entirely abrogate growth of B. burgdorferi sensu lato (262). Consequently, as a necessary quality control measure, each new batch of liquid medium must be tested for its ability to support adequately the growth of laboratory-adapted strains of B. burgdorferi sensu lato. Once prepared, BSK-H medium can be preserved for future use at −20°C for at least 8 months (262).
Cultures in liquid medium are usually incubated at 30° to 34°C under microaerophilic conditions. Incubation at temperatures of 39°C or higher may reduce or prevent growth (17). Cultures are incubated for up to 12 weeks, which is much longer than is necessary to grow most other human bacterial pathogens, due in part to the spirochete's prolonged generation time (7 to 20 h or longer) during log-phase growth (17, 266, 353). Detection of growth is accomplished by periodic examination of an aliquot of culture supernatant for the presence of spirochetes by dark-field microscopy or by fluorescence microscopy after staining with the fluorochrome dye acridine orange or a specific fluorescence-labeled antibody (277, 367). Visualized spirochete-like structures should be confirmed as B. burgdorferi sensu lato by demonstration of reactivity with specific monoclonal antibodies or by detection of specific DNA sequences by using PCR methodology (215, 312, 353, 367). Lack of experience in the microscopic detection of B. burgdorferi sensu lato can lead to false-positive readings, as other structures such as cellular debris may appear thread-like and thus be mistaken for B. burgdorferi sensu lato (110).
B. burgdorferi sensu lato can also be grown on solid media with agarose to solidify the liquid media discussed above and incubated under microaerophilic or anaerobic conditions (17, 158, 266). An advantage of using solid media is that individual colonies can be identified as a means to select out particular clonal strains of B. burgdorferi sensu lato.
Laboratory-propagated strains of B. burgdorferi sensu lato can be successfully cocultivated with tick cell lines (157, 213, 229) and with certain mammalian cell lines (121, 311, 341). Cocultivation techniques may prove to be useful for primary isolation of B. burgdorferi sensu lato from clinical specimens as well (311, 341).
(ii) Culture of clinical specimens.
B. burgdorferi sensu lato can be recovered from various tissues and body fluids of patients with LB, including biopsy (14, 25, 31, 140, 165, 178, 200, 204, 209, 214, 221, 227, 237, 256, 267, 303, 327, 333, 342, 369) and lavage (369) specimens of EM skin lesions, biopsy specimens of ACA skin lesions (14, 258, 267, 342), biopsy specimens of borrelial lymphocytoma skin lesions (188), cerebrospinal fluid specimens (60, 147, 237, 267, 342), and blood specimens (13, 22, 189, 216, 221, 322, 367, 368, 374). Anecdotally, recovery of B. burgdorferi sensu lato from other tissue or fluid specimens (189), such as synovial fluid (289), cardiac tissue (312), and iris (265), has also been reported.
Aside from the late cutaneous manifestation ACA, from which B. burgdorferi sensu lato has been recovered more than 10 years after onset of the skin lesion (14), the vast majority of successful isolations have been from untreated patients with early disease (EM or early neuroborreliosis) (147, 219, 303, 351, 360).
(a) Erythema migrans. Recovery of B. burgdorferi sensu lato from 2- to 4-mm skin biopsy samples of an EM skin lesion can typically be achieved for at least 40% of untreated patients (Table 2). The highest reported success rates were 86% in a study of 21 U.S. patients for whom 4-mm skin biopsy specimens were cultured (25) and 88% in a study from The Netherlands of 57 patients who underwent a 4-mm skin biopsy (342). B. burgdorferi sensu lato can be recovered from both primary (site of tick bite) and secondary (presumed to arise by hematogenous dissemination) EM lesions (204, 213). Despite the clinical tradition of biopsying the advancing border of an EM lesion, a recent systematic study of this question conducted in Europe found comparable yields from biopsy specimens taken from the EM center (140). According to one report, positive cultures can also be obtained from clinically normal-appearing skin 4 mm beyond the EM border (25).
TABLE 2.
Cultivation of Lyme borreliae from clinical samples
| Site | Representative culture yield (%) (reference[s])
|
|
|---|---|---|
| United States | Europe | |
| Skin | ||
| Erythema migrans | >50a (25, 200, 204, 214, 227, 303, 327, 369) | ≥40b (14, 31, 140, 165, 209, 237, 256, 333, 342, 380) |
| Acrodermatitis chronica atrophicans | NDh | ≥22c (14, 258, 342) |
| Borrelial lymphocytoma | ND | 24 (188) |
| Cerebrospinal fluid | ND | 10d (60, 147, 237, 342) |
| Synovial fluid | Anecdotal | Anecdotal |
| Blood | >40e (367, 368) | 1.2f to 9g (13, 189) |
Highest reported yield, 86% (25).
Highest reported yield, 88% (342).
Highest reported yield, 60% (342).
Highest reported yield, 17% (342).
Plasma (high volume, ≥9 ml) of untreated adult United States patients with erythema migrans.
Plasma (low volume, ≤3 ml) of European adults with erythema migrans.
Plasma (low volume, <1 ml) of untreated European children with erythema migrans.
ND, no data available.
B. burgdorferi sensu stricto can also be grown from culture of saline injected into and then withdrawn from an EM lesion. In one study of a group of U.S. patients with EM, cultures were established for both a 2-mm skin biopsy sample and a lavage sample in which nonbacteriostatic sterile saline was injected into the same EM lesion and then recovered using a novel two-needle technique (369). A version of this technique had previously been found to be successful in recovering B. burgdorferi sensu lato from the skin of infected animals (259). In the clinical study, excluding contaminated samples, B. burgdorferi sensu stricto was recovered from 20 (74%) of the 27 skin biopsy specimens, compared with 12 (40%) of the 30 lavage samples (P < 0.05) (369). Although less sensitive, cutaneous lavage has the advantage of being less invasive.
Recovery of B. burgdorferi sensu stricto from solitary EM lesions is significantly more likely in U.S. patients with skin lesions of shorter duration (214), suggesting that the patient's immune response is usually effective in clearing the spirochete from that skin site over a relatively short time interval. Consistent with this premise are the results of a recent study in which the number of spirochetes in 2-mm skin biopsy samples of the advancing border of an EM skin lesion was determined using a quantitative PCR (qPCR) technique (177). This study demonstrated that significantly fewer spirochetes were present in older or larger EM lesions (in the United States the size of an EM lesion correlates directly with its duration) (23, 214, 215). The same phenomenon probably explains a much earlier clinical observation that in the absence of antibiotic therapy, EM lesions will nevertheless resolve in a median time period of approximately 4 weeks (317). Clearance of spirochetes from the skin in the absence of antibiotic treatment has also been documented in a rhesus macaque model of B. burgdorferi sensu stricto infection (254).
Borrelia burgdorferi sensu stricto usually cannot be recovered on culture of EM lesions of patients already receiving appropriate antibiotic treatment (372) and rarely, if ever, cannot be recovered from the prior site of a resolved EM lesion in U.S. patients who have completed a course of appropriate antimicrobial therapy (26, 214). Borrelia burgdorferi sensu stricto has been recovered, however, from cultures of EM lesions of patients treated with the narrow-spectrum cephalosporin cephalexin, an antibiotic which is inactive in vitro against B. burgdorferi sensu lato and ineffective clinically (4, 226).
(b) Whole blood, serum, and plasma. The rate of recovery of B. burgdorferi sensu stricto from blood or blood components of untreated patients with EM had generally been 5% or less (22, 108, 323, 345), and until recently this source of culture material was largely abandoned. In past studies on the sensitivity of blood cultures for recovery of B. burgdorferi sensu stricto, only a very small volume of less than 1 ml of blood or blood components was cultured (22, 108, 216, 217, 322, 323, 345). For other bacterial infections, however, the volume of blood cultured is an important determinant of yield (201, 354, 356). The reason for this is that with more conventional pathogens the number of cultivable bacteria per milliliter of whole blood is less than 1 in 50% of bacteremic patients and less than 0.1 in nearly 20% (82, 151). Therefore, in view of the recommendation to culture quantities of blood as large as 20 to 30 ml for other bacterial infections, the rationale for culturing much smaller volumes for LB patients was open to challenge.
In a series of recent experiments with adult patients with EM from the United States, it was demonstrated that recovery of B. burgdorferi sensu stricto was better from serum than from an identical volume of whole blood (374) and that the yield from plasma was significantly greater than that from serum (367). Yield directly correlated with the volume of material cultured. Recovery of B. burgdorferi sensu stricto from high-volume cultures of 9 ml of plasma inoculated into a modified BSK II preparation devoid of antimicrobials and gelatin, with a 20:1 ratio of medium to plasma, was consistently above 40% (367, 368). Unfortunately, high-volume plasma cultures are not appropriate for young children, since obtaining such a large quantity of blood would be unacceptable. Reported rates of recovery from blood of patients with EM in Europe have been <10% (13, 189). This may be due to a low frequency of hematogenous dissemination of B. afzelii, the principal cause of EM in Europe (351), or to culturing an inadequate volume of blood.
A study that addressed further increasing the volume of plasma from 9 ml to 18 ml for adult U.S. patients with EM found only a small increment of approximately 10% in culture yield (368), suggesting that if substantially greater yields from blood cultures are still possible, it will be by further modifying the culture medium rather than by increasing the volume of plasma cultured. In that study it was estimated that the average number of cultivable B. burgdorferi sensu stricto cells per milliliter of whole blood was approximately 0.1, which, if correct, would explain why blood cultures had such a consistently low yield in former studies in which the volume of blood or blood components cultured was extremely small (368).
A recent study of patients with EM showed a greater recovery of B. burgdorferi sensu stricto from blood of symptomatic patients; nevertheless, the majority of symptomatic patients had negative blood cultures (371), raising doubts about the reliability of the assumption that the presence of symptoms indicates dissemination.
Although less extensively studied, culture of blood samples is rarely positive in patients with any objective clinical manifestation of LB other than EM (191, 216; J. Nowakowski, unpublished observations). Blood cultures are also negative in patients with persistent subjective symptoms following completion of an appropriate course of antibiotic treatment (154, 191).
(iii) Sensitivity of culture.
Although a number of as low as one spirochete can be recovered on culture using laboratory-adapted and continuously propagated strains of B. burgdorferi sensu stricto (17, 254, 262, 331), the sensitivity of culture for clinical specimens is undefined. Compared to PCR for detection of spirochetes in cutaneous specimens, culture has proven to be slightly more sensitive in some studies but not in others (31, 165, 209, 227, 256, 303, 327, 380). Such disparate results are likely to be attributable to differences in the various studies in the PCR protocols employed, including type of PCR and/or primer and target selection and/or method of tissue preservation, as well as differences in culture techniques, including size of the skin biopsy sample cultured and/or choice of culture medium. In a recent U.S. study of skin biopsy samples of 47 untreated adult patients with EM lesions, culture grew B. burgdorferi sensu stricto in 51%, compared with an 81% detection rate using a qPCR method (P = 0.004 by Fisher's exact test, two tailed) and a 64% detection rate using a nested PCR (P = 0.3) (227).
Although all of the different B. burgdorferi sensu lato species and subspecies recognized to cause human infection can be cultivated, it is not clear whether the sensitivity of culture is identical for each. In one study, subtyping of B. burgdorferi sensu stricto was performed directly on 51 skin biopsy samples of patients with EM by using PCR-restriction fragment length polymorphism of the 16S-23S rRNA gene intergenic spacer region and also on the strains of B. burgdorferi sensu stricto that grew from culture of the same biopsy samples (176). No significant difference in the rate of recovery of any single subtype was observed. However, due to the relatively small number of isolates of B. burgdorferi sensu stricto in that study, further investigation of this question is needed.
(iv) Practical considerations.
Culture has been principally used as a diagnostic modality in research studies and has enabled a better understanding of the clinical, laboratory, and pathogenetic aspects of LB through identification of a group of culture-authenticated patients (7, 215, 309, 332, 333). For example, the recognition that substantive differences exist in the clinical manifestations of patients with EM caused by B. burgdorferi sensu stricto compared with those with EM caused by B. afzelii was based on analysis of culture-confirmed cases (332). In addition, culturing of ticks, EM skin lesions, and blood samples was instrumental in demonstrating that different subtypes of B. burgdorferi sensu stricto vary in their potential to spread to extracutaneous sites (304, 370). Patients with culture-confirmed LB have also been a valuable source of specimens to assess the accuracy of other diagnostic tests (7, 203). Such well-defined patient populations have also played an important role in studying therapeutic regimens (375) and vaccines (327).
Culture, however, has not been used in routine clinical practice as a diagnostic test for several reasons. One reason has been the lack of consistent availability of high-quality (i.e., borrelial growth-promoting) lots of liquid medium for growing B. burgdorferi sensu lato. Until fairly recently, this medium was not sold commercially, and although BSK-H is now available commercially, it has periodically been in short supply or of variable quality.
Furthermore, by most conventional bacteriologic standards, borrelial cultures are more labor-intensive, more expensive, and much slower, requiring up to 12 weeks of incubation before being considered negative. The rapidity of identifying a positive culture, however, is directly dependent on the frequency with which the culture supernatant is examined microscopically, since macroscopic changes in the appearance of the culture medium tend to occur later, if at all. In our research studies, in which B. burgdorferi sensu stricto cultures are usually first examined only at 2 weeks, 70% of positive cultures of skin biopsy samples of EM lesions and approximately 85% of positive cultures of plasma from EM patients turned positive at 2 weeks, and approximately 95% of each type of sample turned positive by 4 weeks (G. P. Wormser, unpublished observations). The time to detection of a positive specimen can be reduced to a clinically relevant time frame if cultures are examined on a daily basis (203). For example, the Marshfield Laboratories identified 74 (82%) of 90 positive cultures within the first 7 days of incubation and 32 (35%) within the first 3 days (277). In the experience of that laboratory, the longest time to detection of a positive culture was 16 days. Culture positivity might be detected even more quickly if aliquots of culture supernatant were examined by PCR, rather than microscopy, to detect B. burgdorferi sensu lato DNA (301), and in the future it is conceivable that such an approach might be adaptable to automation.
Another limiting factor is that culture is useful only for untreated patients. Culture positivity is rapidly aborted by even a few doses of appropriate antibiotic treatment (214, 303).
Perhaps the most fundamental limitation is that culture is far too insensitive in patients with extracutaneous manifestations of LB, which is unfortunately the group of patients who pose the greatest diagnostic confusion (219, 314). Culture has been most successful for patients with early LB who have EM, but visual recognition of the characteristic appearance of the skin lesion is usually sufficient for an accurate diagnosis, and no laboratory testing is indicated (218, 219). Culture would be helpful only in a minority of cases in which the rash may be atypical or the patient was not known to have had tick exposure in an area where LB is endemic.
Molecular methods of detection of B. burgdorferi sensu lato.
For laboratory diagnosis of LB, the utilization of molecular techniques has focused mainly on PCR-based methods. The first PCR assay for specific detection of a chromosomally encoded B. burgdorferi sensu lato gene was reported in 1989 (282). Various other PCR protocols were subsequently developed for detection of B. burgdorferi sensu lato DNA in clinical specimens and were reviewed by Schmidt in 1997 (291).
(i) PCR analysis of clinical specimens.
Given that the number of spirochetes in infected tissues or body fluids of patients is very low, appropriate procedures for sample collection and transport and preparation of DNA from clinical samples are critical for yielding reliable and consistent PCR results. A variety of clinical specimens from patients with suspected Lyme disease have been analyzed by PCR assays (291). Of these, skin biopsy samples taken from patients with EM or ACA have been the most frequently tested specimens (346). Depending on the clinical manifestations of the patients, appropriate body fluid samples (e.g., blood, CSF, or synovial fluid) can be collected and analyzed by PCR.
The sensitivity of PCR assays may be reduced by degradation of the B. burgdorferi sensu lato DNA during sample transport, storage, and processing. If the tissue is kept in BSK medium for over 24 h, some spirochetes will have migrated from the skin biopsy to the culture medium. In this case, DNA should be prepared from both the skin biopsy and the medium and analyzed by PCR separately. Comparative analysis using a quantitative PCR assay of skin biopsy specimens placed in BSK overnight to 2 days (177) has demonstrated a higher copy number of B. burgdorferi sensu stricto DNA in samples extracted from the medium than in those extracted from the skin sample. This may be attributed to migration of the spirochetes out of the skin sample and/or to the presence of PCR inhibitors in skin (G. Wang, unpublished data). Alternatively, spirochetes that have migrated into the medium can be collected by centrifugation and subjected to DNA extraction together with the biopsy tissue.
Clinical specimens collected from patients should be subjected to DNA extraction and PCR analysis shortly after collection, or they should be kept frozen. Studies on infected animal tissues suggest that PCR with DNA prepared from fresh frozen tissues has higher yields than that with DNA from paraffin-embedded, formalin-fixed tissues (163).
As host DNA can interfere with PCR detection of B. burgdorferi sensu lato in clinical and tick samples (62, 302), an optimized DNA extraction procedure is essential to yield reliable PCR results for certain clinical samples (28). Also, PCR inhibitors may be present in various biological samples (blood, urine, synovial fluid, and CSF) obtained from patients (31); this can usually be assessed by spiking negative samples with a known number of spirochetes or particular amounts of spirochetal DNA during DNA extraction or PCR amplification. In most cases, inhibition of PCR may be minimized by dilution of the extracted DNA (62).
PCR results can be qualitative (conventional PCR and nested PCR) or quantitative (competitive PCR and real-time PCR). Each of these PCR methods has its advantages and disadvantages. For laboratory diagnosis of B. burgdorferi sensu lato infection, a qualitative PCR is usually sufficient. Nevertheless, several real-time PCR instruments such as the Sequence Detection System (Applied Biosystems, Inc.) and LightCycler (Roche Diagnostics, Inc.) are now commercially available and offer options for automation in a clinical laboratory setting (90).
The efficiency of a PCR assay is determined by several factors. Among these, the selections of an appropriate gene target and primer set for PCR amplification are the most important in development of any new PCR protocols. In general, a PCR primer set yielding an amplicon of 100 to 300 bp is recommended, as it has high amplification efficiency under standard PCR conditions and can reduce the effects of DNA fragmentation during sample processing. Although PCR assays targeting numerous B. burgdorferi sensu lato genes have been employed in research laboratories, only a few of these genes have been utilized by clinical laboratories as targets for PCR analysis of B. burgdorferi sensu lato DNA in clinical specimens. These include chromosomally carried genes such as rRNA genes, flaB, recA, and p66 and the plasmid-carried gene ospA (291).
(a) PCR analysis of skin biopsy samples from patients with cutaneous manifestations. B. burgdorferi sensu lato DNA was first detected by PCR in skin biopsies from three of four patients with EM and four of five patients with ACA in The Netherlands in 1991 (199). In 1992, Schwartz et al. reported on the detection of B. burgdorferi-specific rRNA genes in skin biopsies from EM patients in the United States (303).
PCR targets that have been employed for detection of B. burgdorferi sensu lato DNA in skin biopsy specimens include p66 (31, 210, 211, 344, 358, 359), the 16S rRNA gene (296, 303), fla (177, 234, 256), the 23S rRNA gene (31), the 5S rRNA-23S rRNA gene spacer (279), recA (177), and ospA (209, 279, 327).
The sensitivity of PCR for detection of B. burgdorferi sensu lato DNA in EM lesions is usually high, ranging from 36% to 88% (Table 3). A prospective study in the United States showed that 85 of 132 (64%) skin biopsies taken from EM patients during a phase III vaccine trial were positive by PCR (327). B. burgdorferi sensu lato-specific DNA has been detected in 54 to 100% of skin biopsy samples from patients with ACA in Europe (Table 3). The sensitivity of PCR for detection of B. burgdorferi sensu lato in skin biopsy samples from ACA patients appears to be dependent on the target sequences selected. Rijpkema et al. reported that 15 of 24 (63%) skin biopsies from patients with ACA in The Netherlands were positive by a nested PCR targeting ospA; only 10 of these samples (42%) were positive if a fragment of the 5S-23S rRNA gene intergenic spacer was targeted (279). Results also depended on which genes were used as targets in PCR for skin biopsies from ACA patients in a small study reported from Germany. Four of five patients with ACA were detected by PCR targeting the p66 gene, versus two of five when the 23S rRNA gene was amplified (31).
TABLE 3.
Sensitivities and specificities of PCR assays for detection of B. burgdorferi DNA in different clinical specimens from patients with LBa
| Clinical specimen and region | No. of studies included | Median % sensitivityb (range) | Reported % specificity range | References |
|---|---|---|---|---|
| Skin biopsy | ||||
| EM | 16 | 69 (36-88) | 98-100 | |
| United States | 4 | 64 (59-67) | 98-100 | 177, 301, 303, 327 |
| Europe | 12 | 73 (36-88) | 100 | 31, 165, 209-211, 234, 256, 272, 279, 344, 358, 359 |
| ACA, Europe | 8 | 76 (54-100) | 100 | 31, 199, 209, 210, 256, 279, 344, 359 |
| Blood, plasma, serum | 6 | 14 (0-100) | ||
| United States | 3 | 18 (0-59) | 100 | 108, 172, 345 |
| Europe | 3 | 10 (4-100) | NAc | 78, 233, 234 |
| CSF | 16 | 38 (12-100) | 93-100 | |
| United States | 6 | 73 (25-93) | 93-100 | 134, 150, 172, 180, 223, 239 |
| Europe | 10 | 23 (12-100) | 98-100 | 11, 57, 74, 88, 130, 162, 233, 236, 270, 377 |
| Synovial fluid | 8 | 78 (42-100) | 100 | |
| United States | 4 | 83 (76-100) | 100 | 30, 172, 224, 252 |
| Europe | 4 | 66 (42-85) | 100 | 87, 133, 270, 293 |
Only studies published in MEDLINE-indexed periodicals during the years 1991 to 2003 and those examined by PCR assay for ≥5 cases are included.
Median sensitivity of PCR assays based on included studies. For studies tested with multiple PCR primer sets, the highest sensitivity reported was selected for analysis.
NA, not available.
(b) PCR analysis of blood from patients with LB. B. burgdorferi sensu lato DNA has been detected by PCR in blood samples from patients with EM (108, 234) and early disseminated disease such as neuroborreliosis and carditis (78, 172). In a prospective study of U.S. patients with EM, B. burgdorferi sensu stricto DNA was detected by PCR in 14 of 76 (18.4%) plasma samples (108).
In general, the sensitivity of PCR for detection of B. burgdorferi sensu lato DNA in blood, plasma, or serum samples from patients with Lyme disease is low (Table 3). The low yield could be a reflection of lack of spirochetemia or transient spirochetemia (22), a low level of spirochetes in blood (108), and/or the presence of PCR inhibitors in host blood (2, 62, 345).
In one study, PCR-documented spirochetemia in patients with EM was correlated with symptomatic illness and with the presence of multiple EM lesions (108); multivariate analysis indicated that a high number of systemic symptoms was the strongest independent predictor of PCR positivity (108).
None of 78 patients with post-Lyme disease syndromes (musculoskeletal pains, neurocognitive symptoms, dysesthesia, fatigue, malaise, headache, or sleep disturbance) had detectable DNA in blood specimens, despite a positive Western immunoblot (IB) for immunoglobulin G (IgG) antibodies against B. burgdorferi sensu stricto in 39 of these patients (154).
(c) PCR analysis of CSF specimens from patients with neuroborreliosis. B. burgdorferi sensu lato DNA has been detected by PCR in CSF specimens from patients with a variety of neurological symptoms in the United States and in Europe (Table 3). Lack of a gold standard method to support the diagnosis of neuroborreliosis makes it difficult to assess the performance of PCR with CSF.
The sensitivity of PCR for detection of B. burgdorferi sensu lato DNA in CSF specimens may be dependent on the clinical presentation, CSF white cell counts, disease duration, and whether antibiotic treatment was given. In a study of 60 U.S. patients with neuroborreliosis (16 with early and 44 with late neuroborreliosis), the sensitivity of PCR in CSF was 38% in early and 25% in late neuroborreliosis, and an inverse correlation was found between duration of antimicrobial treatment and PCR results (223). In this study, four different PCR primer or probe sets were used, three targeting OspA genes and one targeting OspB genes, and concordance between the different assays was poor. In a Swedish study, B. burgdorferi sensu lato DNA was detectable only in LB patients with CSF pleocytosis (7/36; 19.4%). None of 29 patients with clinical signs of LB (EM, cranial neuritis, or radiculoneuritis) without CSF pleocytosis was positive by PCR analysis of CSF specimens (236). Another study found that 7 of 14 (50%) neuroborreliosis patients from Denmark with disease duration of less than 2 weeks yielded a positive PCR result, compared with only 2 of 16 (13%) patients in whom the illness duration was greater than 2 weeks (P = 0.045) (165).
(d) PCR analysis of synovial fluid from patients with Lyme arthritis. PCR analysis of synovial fluid is a much more sensitive approach than culture for detection of B. burgdorferi sensu lato in affected joints of patients with Lyme arthritis (30, 87, 133, 172, 224, 252, 270).
In a U.S. study of 88 patients, B. burgdorferi sensu stricto DNA was detected in synovial fluid of 75 (85%) patients with Lyme arthritis (224). The PCR positivity rate was lower in patients who had received antibiotic therapy than in untreated patients. Of 73 patients who were untreated or treated with only short courses of oral antibiotics, 70 (96%) had a positive PCR in synovial fluid samples. In contrast, B. burgdorferi sensu stricto DNA was demonstrated in only 7 of 19 (37%) patients who received either parenteral antibiotics or oral antibiotics for more than 1 month (224). Four PCR primer sets were used in this study, three amplifying DNA sequences encoding OspA and one targeting a portion of the gene encoding 16S rRNA. Among the 75 patients with positive PCR results, the most sensitive primer-probe set detected B. burgdorferi sensu stricto DNA in 89%, versus 56% for the least sensitive set. Only 48 of the 75 (64%) patients with a positive PCR result were positive with all three OspA primers (224). Therefore, the yield of PCR to detect B. burgdorferi sensu lato DNA in synovial samples will depend on the primer-probe set (s) used and the duration of antimicrobial therapy. It is noteworthy that B. burgdorferi DNA has been detected in synovial membrane samples of patients whose synovial fluid specimens were PCR negative after antibiotic treatment (269).
The observation of higher sensitivity of PCR targeting B. burgdorferi sensu stricto plasmid-encoded OspA than of that targeting the 16S rRNA chromosomally encoded genes in synovial fluid specimens (224, 252) has been referred to as “target imbalance.” It has been speculated that B. burgdorferi sensu stricto present in the synovium may selectively shed OspA DNA segments into the synovial fluid.
(e) PCR analysis of urine samples from patients with early Lyme borreliosis. B. burgdorferi sensu lato has been frequently recovered from culture of urinary bladder specimens of experimentally infected laboratory animals (21, 348), suggesting that this spirochete could be excreted into the urine. However, although there have been reports of detection of B. burgdorferi DNA by PCR in urine specimens from patients with EM (28, 164, 290, 292), with neuroborreliosis (130, 146, 164, 172, 187, 270), or with Lyme arthritis (109, 146, 270), the sensitivity was highly variable. In addition, nonspecific amplification in urine PCR using different targets has been documented (31, 146, 172, 290). Thus, it is not appropriate to use urine PCR for the laboratory diagnosis of LB.
(ii) Real-time quantitative PCR.
A real-time PCR assay was first used for quantitation of B. burgdorferi DNA in tissues from experimentally infected laboratory mice (208, 242). Subsequently, it has been employed to analyze the number of spirochetes in field mice (33, 349), dogs (330), field-collected or laboratory-infected tick vectors (103, 260, 347), and clinical specimens of patients with LB (177, 296; reviewed in reference 346). Also, real-time PCR assays have been utilized to genotype the pathogenic B. burgdorferi sensu lato species in both ticks and EM patients in Europe (207, 261, 276). In addition, real-time multiplex PCR assay has been applied for simultaneous detection of B. burgdorferi sensu stricto and Anaplasma phagocytophilum infections in ticks (66).
Recently, the number of spirochetes in clinical specimens of patients with LB was determined by real-time qPCR assays (177, 296). In one study, B. burgdorferi sensu stricto-specific recA DNA was detected by a LightCycler qPCR assay in 40 (80%) skin biopsy samples from 50 untreated adult U.S. patients with EM (177). The number of spirochetes in a 2-mm biopsy ranged from 10 to 11,000, with a mean number of 2,462 spirochetes. Significantly higher numbers of spirochetes were detected in culture-positive than in culture-negative skin specimens (3,940 versus. 1,642 spirochetes; P < 0.01). In another study, B. burgdorferi sensu lato fla was detected using a TaqMan probe in 5 of 28 (17.9%) synovial fluid specimens and 1 of 5 (20%) synovial membrane biopsies obtained from 31 patients with arthropathies in Switzerland (296). The numbers of spirochetes varied from 20 to 41,000/ml of synovial fluid. Of 56 CSF samples from 54 patients with a clinical suspicion of neuroborreliosis, only one (1.8%) was positive by real-time PCR (296). It is not clear whether these CSF specimens were simultaneously analyzed by conventional PCR or any other molecular assays.
(iii) PCR sensitivity and specificity.
Table 3 summarizes the sensitivities and specificities of PCR assays for the detection of B. burgdorferi DNA in different clinical samples as published in MEDLINE-indexed periodicals during the years 1991 to 2003. Of the 24 studies in which B. burgdorferi sensu lato DNA in skin biopsies was examined, the sensitivities of the PCR assays varied from 36 to 88% for patients with EM and from 54 to 100% for patients with ACA. The median sensitivities of the reported PCR assays for detection of B. burgdorferi DNA in skin biopsies from patients with EM and ACA were 69% and 76%, respectively. The sensitivity of PCR assays for detection of B. burgdorferi sensu lato in whole blood (plasma or serum) and CSF specimens is low (Table 3). By contrast, higher PCR sensitivities were reported in both U.S. and European studies with synovial fluid samples from patients with Lyme arthritis.
A published meta-analysis also demonstrated that PCR is a very sensitive approach when it is employed to detect B. burgdorferi sensu lato DNA in skin biopsy and synovial fluid specimens from patients with LB, whereas the diagnostic value of PCR assays for detection of B. burgdorferi sensu lato DNA in blood (plasma or serum) and CSF specimens is low (85).
(iv) Applications and limitations of molecular methods.
PCR-based molecular techniques have been employed for (i) confirmation of the clinical diagnosis of suspected LB, (ii) molecular species identification and/or typing of the infecting spirochetes in clinical specimens or on cultured isolates, and (iii) detection of coinfection of B. burgdorferi sensu lato and other tick-borne pathogens. However, PCR assays have not been widely accepted for laboratory diagnosis of LB because of low sensitivity in CSF and blood. PCR as a diagnostic tool may be hampered by potential false-positive results due to accidental contamination of samples with a small quantity of target DNA. False-positive PCR results have been reported for LB (206).
Although PCR is highly sensitive for detection of B. burgdorferi sensu lato DNA in skin biopsy samples from patients with EM (85), such testing is rarely necessary, as a clinical diagnosis can be easily made if the characteristic skin lesion is present. For patients with LB involving systems other than skin, PCR sensitivity is in general low, with the exception of patients with Lyme arthritis.
Immunologic Diagnosis of B. burgdorferi Sensu Lato Infection
The complexity of the antigenic composition of B. burgdorferi sensu lato has posed challenges for the serodiagnosis of LB. As described above, a sizable number of antigens are differentially expressed in the vector and the host (103, 300), and some are exclusively expressed in vivo in the infected mammalian host (72, 94). Furthermore, antigenic differences exist among the B. burgdorferi sensu lato species causing LB (117, 135, 281, 336).
Due to limitations in direct detection of B. burgdorferi sensu lato in clinical specimens, antibody detection methods have been the main laboratory modality used to support a clinical diagnosis of LB. Although a cellular immune response is also elicited, most methods used in the laboratory confirmation of LB involve detection of serum antibodies. It is, however, important to emphasize that relatively few studies have evaluated serologic tests in culture-confirmed populations. Therefore, conclusions derived from published studies on the serology of patients with clinically defined Lyme borreliosis, discussed later in this review, have inherent limitations.
B. burgdorferi sensu lato antigens of importance in immunodiagnosis.
It is important to understand the antigenic composition of B. burgdorferi sensu lato, as it pertains to immunodiagnosis. Numerous early studies recognized the importance of the flagellar protein flagellin (41 kDa), or FlaB, as an immunodominant antigen (63, 70). Strong IgG and IgM responses to this protein are developed within a few days after infection with B. burgdorferi sensu lato (8, 84, 111). Thus, some immunoassays consist of purified flagella alone (114, 115, 138, 148), whereas in others, flagellin is added to enrich the antigenic mixture (174). Unfortunately, although highly immunogenic, this antigen is highly cross-reactive with antigens in other bacteria, particularly when denatured, as in immunoblots (35, 91, 179). Certain flagellin epitopes are also cross-reactive with antigens found in mammalian tissues such as neural tissues, synovium, and myocardial muscle (1, 179). The internal portion of the flagellin molecule, containing the variable, genus-specific immunodominant domain, is less cross-reactive with antigens of other bacteria than the whole protein (101, 179, 274). The flagellar outer sheath protein FlaA, with a molecular size of 37 kDa, is another immunodominant antigen, especially in early disease (7, 84, 104, 246).
One of the most immunodominant antigens early after infection with B. burgdorferi sensu lato is the plasmid-encoded OspC protein (molecular size of about 21 to 25 kDa) (8, 84, 89, 241, 363), which begins to be expressed during tick feeding while the spirochete is still in the tick midgut. Highly passaged in vitro-cultured B. burgdorferi sensu lato does not express OspC, which explains why the importance of this antigen was unrecognized in early studies that used high-passage B. burgdorferi sensu lato as the source of antigen (63, 70). OspC is heterogeneous, and amino acid differences exist among the sequences of OspC proteins from the different B. burgdorferi sensu lato species (135, 197, 336). Furthermore, intraspecies differences also exist; for example, at least 13 OspC serotypes have been identified in B. garinii by using a panel of monoclonal antibodies (361). Sixty-nine ospC groups have been described among B. burgdorferi sensu lato species isolated from different sources in Europe and United States when assayed using the technique of single-strand conformational polymorphism (159). Of interest is that invasive B. burgdorferi sensu lato strains appear to belong to just 24 of the 69 ospC groups (159). It is postulated that OspC is an important virulence factor for both infectivity and invasiveness of B. burgdorferi sensu lato species (86, 193, 304). Antibodies elicited against OspC may be borreliacidal and thus may play a role in the functional antibody assays described below. The search for immunogenic, conserved epitopes within the OspC protein has led to the development of a synthetic peptide that contains the conserved C-terminal 10 amino acids of OspC (pepC10) (198).
The chromosomally encoded 39-kDa protein BmpA (306) is also immunogenic. The gene encoding this protein is located in a bmp cluster that also includes genes for BmpB, BmpC, and BmpD; all have molecular sizes similar to that of BmpA (271). However, it is unclear if BmpB through -D have any utility as antigens in serologic assays. Genetic and antigenic differences have been found among BmpA sequences of different B. burgdorferi sensu lato species, which may limit the use of this antigen in serologic testing (281).
Decorin binding protein A (DbpA) (also referred to as Osp17), which has a molecular size of approximately 17 kDa, is immunogenic (124, 136). DbpA is associated with binding of B. burgdorferi sensu lato to the host collagen-associated proteoglycan decorin. Similar to the case for other B. burgdorferi sensu lato proteins, considerable differences in amino acid sequences exist among DbpA proteins of different B. burgdorferi sensu lato species.
Neither OspA (31 kDa) nor OspB (34 kDa) is significantly expressed by B. burgdorferi sensu lato during early stages of infection. OspA is down-regulated in the tick midgut during tick feeding (298, 299). Presumably OspA and OspB are eventually expressed in mammals, since antibodies to these antigens can be detected during late infection (10, 84). Antibodies to OspA or OspB may be borreliacidal (46, 132, 286, 287). OspA antibodies were readily generated after administration of the recombinant OspA vaccine, which was commercially available in the United States for use in humans until March 2002 (327).
Recently, the Vmp-like sequence expressed (VlsE) protein, a surface-exposed lipoprotein encoded by the linear plasmid lp28-1 of B. burgdorferi B31, has been found to be highly immunogenic (378). Antigenic variation in B. burgdorferi sensu lato occurs when recombination between silent vls cassettes and vlsE takes place. VlsE in B. burgdorferi sensu lato has a predicted molecular mass of approximately 34 to 35 kDa and contains variable and invariable domains. Studies on the antigenicity of this protein demonstrated that one invariable region (IR6), which is found within the variable portion of VlsE, is highly immunogenic (161, 170). This sequence is conserved among B. burgdorferi sensu lato species, making it an appealing candidate as a broadly reactive immunodominant antigen.
Several other antigens expressed in vivo in the mammalian host appear promising in the search for highly specific immunodominant antigens. Among these are the P35/BBK32 and P37 proteins (10, 94, 96). BBK32 is a fibronectin binding protein first described as P35 in a form lacking some of its N-terminal amino acids; it has an apparent molecular mass of about 47 kDa (96). The in vivo-expressed P35/BBK32 and P37 proteins are not equivalent to other B. burgdorferi sensu lato proteins of diagnostic significance of similar size, such as VlsE, FlaA, or the P35 scored by Engstrom et al. in their immunoblot evaluation (89). Expression of P37 or P35/BBK32 by B. burgdorferi sensu lato in clinical materials, as well as in ticks during a blood meal, has supported their presence in the early stages of infection (80, 96).
Antibody detection methods.
Several methods have been used for detection of antibodies to B. burgdorferi sensu lato. Early modalities included indirect immunofluorescent-antibody assays (IFA) and a variation of this assay using antigens attached to a membrane (FIAX) (251). These assays have for the most part been replaced by EIA, including enzyme-linked immunosorbent assay (ELISA) and enzyme-linked fluorescent assay (ELFA), that are more amenable to automation (366). Additional immunoassays in use are Western IBs and immunochromatographic and dot blot assays. Methods less frequently used are assays that detect borreliacidal (functional) antibodies, antibodies bound to immune complexes (IC), and hemagglutinating antibodies (46, 48, 248, 287).
At least 70 different commercial immunoassays to detect B. burgdorferi sensu stricto antibodies have been approved for use in the United States by the Food and drug Administration (FDA) (6, 55). Several of these assays are sold under a label different from that under which they were originally marketed, and others are no longer available. Most of these assays use the B31 type strain of B. burgdorferi sensu stricto as the source of antigen. Other strains of B. burgdorferi sensu stricto have been used in assays developed by academic centers in the United States, and various B. burgdorferi sensu lato species have been employed in in-house or commercial assays available in Europe.
(i) IFA.
IFA uses cultured organisms fixed onto glass slides. Serum specimens are diluted in preparations that may include an absorbent such as material derived from Reiter treponema or egg yolk sac to remove nonspecific antibodies. After addition of fluorescein isothiocyanate-labeled anti-human IgG or IgM, the presence of antibodies is detected by fluorescence microscopy. Specimens testing reactive at screening dilutions are serially diluted, and titers of 128 or 256 for IgM or IgG, respectively, are usually considered positive (186, 284). Limitations of this assay include the need for fluorescence microscopy and for well-trained personnel and the subjectivity in reading and interpreting fluorescence microscopy. These issues were addressed in the modification of this assay that was available about a decade ago (FIAX; Whittaker), which used antigens applied to membranes, with the degree of fluorescence read by an automated system.
(ii) Enzyme immunoassays.
ELISA is the most frequently used format to test for antibodies to B. burgdorferi sensu lato. Most commonly, antigen mixtures comprised of whole-cell sonicates of B. burgdorferi sensu lato are used as the source of antigen for the detection of IgG, IgM, or IgA antibodies individually or in combination (most frequently IgG-IgM combinations). Purified antigens, such as flagellar components, or recombinant antigens, such as P39, have been added to the antigen mixture in some kits (6). Recently, an ELISA using only a single synthetic peptide derived from the VlsE sequence (IR6 or C6 peptide) as the source of antigen has become commercially available.
Using sonicated whole-cell preparations of low-passage B. burgdorferi sensu lato, the sensitivity of ELISA is in general less than 50% in acute-phase sera of patients with EM of a duration of less than 1 week. Sensitivity increases rapidly over time after the first week in untreated patients with EM. Sensitivity is also high in patients with EM who are symptomatic or who have multiple EMs. Sensitivity is very high in patients with objective evidence of extracutaneous involvement (e.g., carditis or neuroborreliosis) (84). ELISA is almost invariably positive in sera of patients with late disease such as arthritis (Table 4) (84).
TABLE 4.
Reactivities obtained using different methods to detect antibodies to B. burgdorferi in Lyme borreliosis in patients from the United States
| Test | % Reactivity (reference[s]) in patients with:
|
|||
|---|---|---|---|---|
| EM, acute phase | EM, convalescent phasea | Neurological involvement | Arthritis | |
| Whole-cell ELISA | 33-49 (7, 8, 89, 337) | 76-86 (7, 8, 89, 337) | 79 (IgG ELISA) (84) | 100 (IgG ELISA) (84) |
| IgM IBb | 43-44 (7, 89) | 75-84 (7, 89) | 80c (84) | 16c (84) |
| IgG IBb | 0-13 (7), 43.6d | 15-21 (7), 80d | 64-72 (84) | 96-100 (84) |
| Two-tier testing | 29-40 (7, 15, 89, 227, 337) | 29-78 (7, 15, 89, 227, 337) | 87 (15) | 97 (15) |
One of the limitations of ELISA for detection of B. burgdorferi sensu lato antibodies is lack of standardization. Variations exist between assays in terms of antigenic composition and in the detection of specific immunoglobulin classes, particularly in the detection of IgM antibodies (7, 8, 34, 167, 337). Such variations may occur among different commercial kits as well as between lots of the same kit. Unlike serological assays for detection of antibodies to human immunodeficiency virus, B. burgdorferi sensu stricto antibody assays cleared by the FDA have not been standardized against a panel of well-characterized sera. The regulatory process for clearance of B. burgdorferi sensu stricto serological assays requires only that the manufacturer provide information demonstrating that the new test is substantially equivalent to a test already approved by the FDA (34).
Whole-cell antigen preparations lack specificity because of the presence of cross-reacting antigens of B. burgdorferi sensu lato. These include common bacterial antigens such as heat shock proteins, flagellar antigens, and others (35, 64, 89, 91). Specificity is also affected by the choice of absorbent material used to dilute the serum specimens. Sera of individuals who received OspA vaccination may react in ELISA using whole-cell sonicates (9). Although the commercial availability of the vaccine has been discontinued, some previously vaccinated individuals may still have antibodies reacting with OspA. Specificity is in general better with substitution of selected recombinant or peptide antigens for whole-cell sonicates.
ELISA has advantages over other immunoassays, including ease of testing, objective generation of numerical values that correlate in relative terms with the quantity of antibodies present, and the capability of automation. Due to lack of specificity of ELISA and other first-tier assays, such as ELFA or IFA, using whole-cell antigen preparations, a positive test is not indicative of seropositivity. A recent analysis of results obtained with an automated ELFA found that the specificity was 91% for 559 samples from a control population (137). Serum specimens testing positive or equivocal with a sensitive ELISA, ELFA, or IFA should be retested with IB; this approach is termed two-tier testing (53). First-tier assays should detect both IgG and IgM antibodies to B. burgdorferi sensu stricto, and separate IgG and IgM IBs should be done as the second tier.
(iii) Western IB.
The use of antigens separated by molecular size in IB assays has contributed to the determination of which antigens of B. burgdorferi sensu lato are immunodominant at different stages of LB. Academic centers in the United States have evaluated in-house IB assays using B. burgdorferi sensu stricto strains other than B31 (84, 89). The few commercial IB assays that are currently available in the United States use B. burgdorferi sensu stricto strains; two manufacturers use B31 (6). Various B. burgdorferi sensu lato species and strains isolated from different geographic locations have been studied in Europe (117, 118, 280). Based on recognized inter- and intraspecies differences among the immunodominant antigens of B. burgdorferi sensu lato, it is not surprising that the source of antigens in IB affects the detection of antibodies in European patients with LB (280).
Whether B. burgdorferi sensu lato antigens elicit IgM versus IgG antibodies depends on the duration of infection and the manifestation of LB. Detection of reactivity is also affected by the quality of the antigen used in the immunoassays (including the type and source of antigen). In early LB, IgM antibodies are directed to OspC and the flagellar antigens, FlaB (41 kDa) and FlaA (37 kDa) (7) (Fig. 1, left). Variable rates of IgM response to BmpA (39 kDa) have been observed in sera of patients with early LB, which appears to be related in part to the source of antigen used and/or the duration of disease prior to testing for antibodies (7, 84, 89, 181). The highest rates for IgM reactivity to the 39-kDa protein were reported by Engstrom et al. (89), using B. burgdorferi sensu stricto strain 297 (84%), and by Ma et al. (181), using B31 (50%). In contrast, Dressler et al. (84), using B. burgdorferi sensu stricto strain G39/40, reported IgM reactivity to the 39-kDa protein in only 4 and 8% of acute- and convalescent-phase sera of patients with EM, respectively. The infrequency of reactivity to BmpA antigen reported by Dressler et al. could be attributed to the low expression of this antigen by the B. burgdorferi sensu stricto G39/40 strain used in their study. We have observed IgM reactivity to this antigen in only 3% of acute-phase sera of patients with EM of a duration of less than 1 week and in 35% of acute-phase sera of untreated patients with EM of a duration of more than 7 days, using a commercial IB kit prepared with the B. burgdorferi B31 strain; convalescent-phase sera from the same groups showed IgM reactivity to the 39-kDa protein in 37% and 31%, respectively (7).
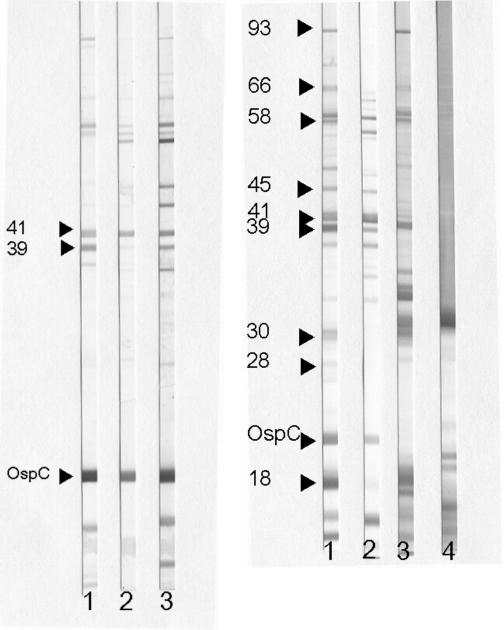
FIG. 1.
(Left) Selected IgM immunoblot reactivities. Lane 1, serum band locator control showing several bands, including the significant 41-kDa protein, 39-kDa protein, and OspC (arrows). Lane 2, serum sample from a patient with early LB with EM. Lane 3, serum sample from a patient with early disseminated LB with multiple EM lesions. Note the larger number of bands observed in serum of the patient with early disseminated LB. (Right) Selected IgG immunoblot reactivities. Lane 1, serum band locator control showing several immunoreactive bands, including those considered significant in the IgG blot criteria (arrows). Lane 2, serum sample from a patient with early disseminated LB with neurological involvement. Lane 3, serum sample from a patient with Lyme arthritis. Lane 4, serum sample from an individual who received three doses of OspA vaccine. In lane 4, note the strong reactivity with OspA (31 kDa) and other antigens below OspC.
The number of antigens recognized in IgM IB and the intensity of the immune response as determined by band intensity are greater in sera of symptomatic patients with EM, those with multiple EMs, or those with EM of a duration of more than 2 weeks at presentation, compared to asymptomatic patients with a solitary EM of a duration of less than 2 weeks (7) (Fig. 1, left). An expanded immunologic response is also found in patients with early neuroborreliosis (84).
In early LB, IgG antibodies are directed to OspC and flagellin (41 kDa). IgG reactivity to BmpA (39 kDa) was reported by Engstrom et al. to occur in 85% of acute-phase sera of patients with EM (89). In our experience, antibody to this antigen is detected in 35% of acute-phase sera of untreated patients with EM of a duration of more than 7 days and in 33% and 64% of convalescent-phase sera of treated patients with EM of durations of less than 7 days and more than 7 days, respectively (7). Other antigens that elicit IgG immunoreactivity detectable by IB prepared with B. burgdorferi B31 are the 93 (also referred as P83/100)-, 66-kDa, 45-, 35-, 30-, and 18-kDa antigens. IgG antibodies reacting with a large number of antigens are typically seen in sera of patients with neuroborreliosis or late LB (84, 280) (Fig. 1, right).
In an attempt to standardize serologic diagnosis of LB, criteria for IB interpretation have been established in the United States (53). These guidelines were derived from the systematic evaluation of IB in LB by two academic centers. The IgM criteria adopted were those of Engstrom et al. (89) based on a study of patients with EM using B burgdorferi sensu stricto strain 297. According to these criteria, a positive IgM blot is defined by the presence of two of three particular immunoreactive bands (OspC, 41 or 39 kDa). The IgG criteria are based on a study by Dressler et al. (84), who used B. burgdorferi sensu stricto isolate G39/40 as the source of antigen for evaluation of sera from patients with various manifestations of LB. These criteria require the presence of at least 5 of 10 particular bands (93, 66, 58, 45, 41, 39, 30, 28, 21 [OspC], or 18 kDa). The guidelines for IB interpretation further state that IgM or IgG criteria can be used during the first 4 weeks of illness, but only IgG criteria can be used after 4 weeks after onset of disease.
Guidelines for IB interpretation in Europe have recently been published, but consensus on criteria has not been reached (118). Criteria applicable to each species causing LB may be needed in Europe (280). IB studies using members of each of the three different B. burgdorferi sensu lato species causing LB in Europe as a source of antigen to test sera from German patients with various manifestations of LB indicated that the overall highest sensitivity was achieved with B. afzelii pKo (118). Levels of immunoreactivity are also a function of the specific manifestation of LB in Europe. For example, OspC of B. garinii strains has better immunoreactivity than OspC of other B. burgdorferi sensu lato species in detecting IgM antibodies in patients with neuroborreliosis. This is most likely explained by the fact that B. garinii is the species that most frequently causes neuroborreliosis in Europe (197).
In general, IB and ELISA have similar sensitivities except in the detection of acute-phase antibodies in early LB (7, 8). In a study of well-characterized sera from 46 culture-confirmed U.S. patients with EM, IgM IB was positive in 43% of acute-phase sera, compared with 33% by whole-cell ELISA (7). IB and ELISA have similar sensitivities when sera of patients with extracutaneous manifestations or late stages are tested (Table 4). The specificity of IB is greater than that of ELISA, as interpretation of IB relies on the presence of specific immunoreactive bands; nevertheless, it is important to emphasize that the specificity of IB is not 100%. Sera of individuals who received the recombinant OspA vaccination may show several bands on IB, depending on the source of antigen used in IB. Most frequently there is IgG reactivity to the 31-kDa antigen (OspA) and to other fragments of this antigen migrating below OspC (Fig. 1, right). These latter bands might be confused with the 18-kDa antigen included in the IgG criteria for IB interpretation (9, 205).
Major limitations of IB include the visual scoring and subjective interpretation of band intensity that may lead to false-positive readings, the cost, and the variability of antibody responses in patients with the same clinical manifestation of LB. False-positive readings are particularly seen in IgM IB due to the presence of low-level reactivities to the 41-kDa and OspC antigens in sera from individuals presenting with other infectious and noninfectious illnesses (84, 89). False-positive IgM IB results have been reported in 6% of patients with illnesses such as rheumatoid arthritis, infectious mononucleosis, and systemic lupus erythematosus (89). Furthermore, IgM reactivity may persist for prolonged periods of time after treatment of early Lyme borreliosis (7). In addition, there is lack of standardization of the antigen source and preparations used in IB. Important methodological considerations include the use of appropriate positive control sera. Alternatively, the use of monoclonal antibodies raised to immunodominant antigens may assist in band location and interpretation. In the absence of an objective means of determining band intensities (densitometry), the use of intensity cutoff materials (weak control sera) is recommended.
(iv) Two-tier testing.
The use of two-tier testing for serodiagnosis of LB was an attempt to improve test accuracy in the United States. It has increased the specificity of B. burgdorferi sensu stricto antibody testing while slightly decreasing the sensitivity, particularly when testing sera of patients with EM (7, 167, 337). Relatively few studies using currently available commercial tests have evaluated the performance of the recommended two-tier testing on well-characterized sera from patients with extracutaneous manifestations of LB. Comparison of sensitivities and specificities between studies is difficult due to the use of different antigen preparations and test methods and inclusion of sera from LB patients with undefined disease duration and treatment history.
The most comprehensive study on the two-tier approach evaluated sera of 280 patients with various manifestations of LB (15). A sensitivity of 38% was observed for sera of patients with EM during the acute phase, which increased to 67% during convalescence after antimicrobial treatment (15). The sensitivity increased to 87% in sera of patients with early neuroborreliosis and to 97% in those with Lyme arthritis (Table 4). In this study specificity was set at 99%. Similar results were obtained for 47 patients with clinically defined EM, for whom the sensitivities of the two-tier test were 40.4% in acute-phase sera and 66% during the convalescent phase after treatment (Table 5) (227).
TABLE 5.
Comparison of the sensitivities of various diagnostic methods for 47 adult patients with erythema migrans evaluated in 2000 at the LB Diagnostic Center in Valhalla, N.Y.a
| Diagnostic method | Sensitivity (%) |
|---|---|
| Culture | |
| Skin biopsy of EM | 51 |
| Plasma | 45 |
| PCR on skin biopsy of EM | |
| Nested | 64 |
| Quantitative | 81 |
| Two-tier serologic testing | |
| Acute phase | 40 |
| Convalescent phase | 66 |
Modified from reference 227 with permission of the publisher.
The restriction of use of the IgM IB criteria to the first 4 weeks of early disease is directed mainly at increasing the specificity of IB, since IgM may persist for prolonged periods of time after treatment and after resolution of symptoms (7). Furthermore, a positive IgM IB using current guidelines for IB interpretation may be found in asymptomatic individuals living in areas of endemicity or in patients with non-Lyme disease illnesses (308). This time-restricted use of the IgM IB may, however, reduce the sensitivity of two-tier testing for confirmation of seroconversion in treated patients evaluated slightly beyond the 4-week time point in early convalescence, before IgG antibodies have fully developed (337).
Newer EIA antibody tests.
Because of the above-described limitations of current ELISA and IB testing, there is interest in developing simplified but accurate new approaches for serodiagnosis. The principal focus has been on the use of purified, recombinant, or synthetic peptides as the source of antigens in immunoassays. Unfortunately, so far no single antigen has demonstrated sufficient sensitivity and specificity to warrant replacing two-tier testing. As mentioned earlier in this report, antigenic variability among B. burgdorferi sensu lato species and the temporal appearance of antibodies to different antigens at various stages of LB make the choice of a single antigen a difficult task.
(i) Enzyme immunoassays using recombinant antigens.
Several immunoassays using recombinant antigens have been developed and evaluated for the serodiagnosis of LB. Recombinant antigens have included those containing the internal portion of the flagellin (P41-G or 41-i), as well as FlaA, BBK32, P39, P35, and outer surface proteins A, B, C, E, F, VlsE, and DbpA (92, 94, 97, 100, 102, 119, 122-124, 144, 160, 182-184, 235, 241, 244, 275, 294).
In serodiagnosis, recombinant antigens have been used alone or in combination. Particularly in Europe they have been prepared from different B. burgdorferi sensu lato species and used in both ELISA and IB formats in an attempt to increase sensitivity. The performance of selected immunoassays using recombinant antigens is shown in Table 6.
TABLE 6.
Selected recombinant and peptide antigens used for detection of IgM, IgG, or polyvalent antibodies to B. burgdorferi
| Recombinant antigen or peptide | Location | Range % positive in the indicated LB disease stage
|
References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EM
|
Neurological
|
Late
|
|||||||||
| IgM | IgG | Polyva | IgM | IgG | Polyv | IgM | IgG | Polyv | |||
| OspC | United States | 41-73 | 35-43 | 100, 102, 183, 184, 241 | |||||||
| Europe | 35-44 | 5-35 | 47-53 | 6-33 | 7-53 | 3-60 | 144, 196, 244, 275 | ||||
| pepC10 | United States | 40-53 | 53 | 9 | 15 | ||||||
| Europe | 36 | 5 | 45 | 8 | 0 | 0 | 196 | ||||
| P41 internal fragment | United States | 43-53 | 35-52 | 183, 184 | |||||||
| Europe | 49 | 34-60 | 119, 144, 273 | ||||||||
| VlsE | United States | 19-40 | 44-63 | 63 | 73 | 100 | 100 | 39 | 97 | 87 | 15, 161, 185 |
| Invariable region 6 (IR-6, C6 peptide) | United States | ||||||||||
| Acute | 45-74 | 60-95 | 94-100 | 15, 171, 255 | |||||||
| Convalescent | 70-90 | 64 | 83-98 | 122, 169 | |||||||
| Europe | 87 | ||||||||||
| FlaA (37 kDa) | United States | 45-68 | 15 | 104 | |||||||
| Europe | 27 | 58 | 74 | 37 | 79 | 246 | |||||
| DbpA | Europe | 9 | 17 | 100 | 93-98 | 122, 123 | |||||
| BBK32 | Europe | 13 | 74-100 | 100 | 96-100 | 122, 123, 160 | |||||
| P66 | United States | 80 | 24 | 50 | 57 | 35-63 | 75-100 | 228 | |||
Polyv, polyvalent enzyme immunoassay.
In general, preparations containing recombinant OspC have performed better with sera of patients with early LB and when IgM antibodies are tested (100, 196). Padula et al., using a recombinant OspC derived from a B. burgdorferi sensu stricto strain, found that it detected IgM antibodies in 62% of U.S. patients with EM (241). However, some European studies have shown lower (30 to 44%) IgM reactivity to recombinant OspC in sera of patients with EM than was found in U.S. studies (196, 244).
Studies using the recombinant internal region of the flagellin protein have demonstrated it to be less sensitive than purified whole flagellin preparations when testing sera from patients with any stage of LB (119). Moreover, although it shows less cross-reactivity than the whole protein when testing sera from non-LB patients, the internal region of this protein still suffers from this limitation (183, 184, 273).
Assays using recombinant VlsE have shown that it has sensitivity comparable to that of recombinant OspC during early disease and superior sensitivity in sera of patients with neuroborreliosis or late manifestations of LB (15, 161, 185). Recombinant VlsE binds IgM antibodies more frequently than the VlsE invariable region 6 (IR6, C6 peptide) in sera of patients with EM (but IgG anti-C6 is already present in patients with EM) or early neuroborreliosis (161, 171, 185).
Recombinant DbpA preparations obtained from different B. burgdorferi sensu lato species have been evaluated in Europe to detect antibodies in patients with LB (61, 122, 124). These recombinants detect mostly IgG antibodies and have the greatest sensitivities (93 to 100%) in sera from patients with neuroborreliosis and late stages of LB. They performed poorly with sera from European patients with EM (124).
European studies have shown that recombinant BBK32 shows high sensitivity (74 to 100%) in the detection of antibodies at any stage of LB, including EM (122, 123, 160). Similar to DbpA, however, this antigen mostly binds IgG antibodies. Epitopes not included in these recombinant antigens may perhaps bind IgM antibodies, since the BBK32 antigen is expressed in vivo during the early stages of infection.
Use of recombinant P66 in the serodiagnosis of LB in the United States has shown promising results. The 66-kDa protein is a candidate ligand for β3-chain integrins with apparent surface localization that is recognized by sera of patients with LB; IgG reactivity to the 66-kDa protein is one of the significant bands included in the IgG IB criteria (84). The central portion of this protein is involved in integrin recognition, while the C terminus contains a surface-exposed immunodominant loop. Studies using cloned segments of this protein as an antigen source in immunoassays show that different portions of P66 protein appear to be preferentially recognized by sera from patients with different manifestations of LB (228). Sera from patients with late disease manifestations most frequently had IgG antibodies to the C terminus. Specificities for IgM and IgG antibodies to full-length P66 were 94% and 91%, respectively (228).
(ii) Peptide-based immunoassays.
The generation of peptides containing selected immunoreactive epitopes of immunodominant protein antigens has led to the evaluation of pepC10 and C6 (IR6). The advantage of these two peptides is that they are highly conserved among different B. burgdorferi sensu lato species and should be less cross-reactive than the full-length antigens.
(a) pepC10 peptide. pepC10 peptide has been evaluated both in Europe (196) and in the United States (15). This peptide preferentially binds IgM antibodies. The reported sensitivity in patients with EM was 40%, increasing to 53% in patients with early neuroborreliosis (15). This peptide does not detect antibodies in patients with late LB (Table 6). Reported specificities of pepC10 are 92 to 99% (15, 196). The presumed advantage of pepC10 over recombinant OspC is that pepC10 is less heterogeneous than recombinant OspC, suggesting that it should be more broadly reactive in samples of patients with LB from different geographic locations, particularly in Europe.
(b)C6 peptide (IR6). The discovery of IR6, a highly immunogenic and highly conserved region of VlsE, brought great optimism to the prospect of finding a single-tier assay for serodiagnosis of LB. Although a C6 peptide assay is now commercially available, most studies published to date have used in-house assays rather than the commercially available test. Published studies have shown that despite not eliciting an IgM response, C6 peptide assays have high sensitivity in all stages of LB. The sensitivity of the C6 assay surpasses that of VlsE in patients with EM (IgG positivity in up to 90% versus 63%) (171, 185). Recombinant VlsE, however, may detect antibodies from patients with neuroborreliosis more frequently than C6 (15, 161). Using a kinetic ELISA, Bacon et al. found that IgG antibodies to recombinant VlsE were detected in 15 of 15 patients with acute neuroborreliosis, compared with 9 of 15 (60%) for IgG antibodies to C6 and 13 of 15 (87%) for two-tier testing (15). Whether additional epitopes present in VlsE but not contained in C6 are needed to detect antibodies in acute neuroborreliosis is currently unknown. Further investigation into the performance of C6 in sera of patients with neuroborreliosis is warranted.
Overall similar sensitivities were found by Bacon et al. for an in-house synthetic peptide C6 assay and two-tier testing with 280 serum samples from patients with various manifestations of LB (66% versus 68%, respectively) (15).
Assays using C6 peptide have also been evaluated in Europe on sera obtained from 23 culture-confirmed EM patients (10 from Austria and 13 from Italy) and on 41 sera from patients with late manifestations of LB (21 from Austrian patients with ACA and 20 from Italian patients with late neuroborreliosis). C6 seroreactivity was observed in 20 of 23 (87%) sera from patients with EM, in 20 of 21 (95%) sera of Austrian patients with ACA, and in 14 of 20 (70%) sera of Italian patients with late neuroborreliosis (169). Fifty-one of 52 (98%) German children with Lyme arthritis tested positive for C6 antibodies in another study (122) (Table 6). These findings further support the notion that C6-based enzyme immunoassays are broadly reactive in sera of patients with LB from different geographic localities.
It has been claimed that the C6 ELISA can be used to assess the outcome of therapy for LB. Philipp et al. reported that 80% of a subset of patients treated for early localized or disseminated LB had a ≥4-fold decrease in their reciprocal geometric mean titers to C6 at 6 months or thereafter (255). Other studies have not confirmed these findings. Peltomaa et al. found that 33% and 86% of patients with early and late LB, respectively, had a <4-fold decline in C6 titers (250). Those authors also reported that in another group of patients, 50% of those with early LB and 83% of those with late LB still had detectable C6 reactivity 8 to 15 years after treatment. Likely explanations for the discrepancies in the findings of Philipp and Peltomaa include differences in serum dilutions used to calculate the decline in antibodies, whether patients had sufficient titers at baseline to permit detection of a fourfold decline at least 6 months later, and/or the patient populations studied (250, 255).
(iii) Use of a combination of recombinant or peptide antigens in immunoassays.
Since no single antigen appears to have the desirable sensitivity to be used alone in the serodiagnosis of LB, some authors have evaluated their use in combination. Rauer et al. (275) investigated the combination of recombinant OspC and P41-i in sera of patients with early LB. Detection of IgM antibodies by the hybrid ELISA was 46%, similar to the sensitivity obtained with whole-cell ELISA (45%) using the B. afzelii pKo strain (275). Other combinations that have been evaluated include P41-i and a 59-kDa fragment of P83 (273) and P41-i, recombinant OspC, and recombinant P83 (144). The latter three-antigen combination showed a sensitivity of greater than 90% in sera of patients with neuroborreliosis.
An immunochromatographic assay that includes recombinant, chimeric, truncated forms of OspA, OspB, OspC, P93, and flagellin as the antigen is currently commercially available in the United States to test for B. burgdorferi sensu stricto antibodies in serum or whole blood (107). This assay is being promoted as a method for first-step testing that can be performed in the health care provider's office. A specificity of 85% was obtained with sera of syphilitic patients with this ELISA (107). Since it includes OspA, this assay would be expected to show reactivity with sera of individuals who have received OspA vaccination. The sensitivity of an ELISA with the same antigen mixture was 44%, compared with 39% for a commercial whole-cell ELISA, in a study of sera from 41 patients with culture-confirmed EM; sensitivities of 62% and 54% for the chimeric versus the whole-cell ELISA were observed with sera of patients with early disseminated LB (patients with multiple EMs or EM plus objective signs of neurologic or cardiac involvement), respectively. A sensitivity similar to that of whole-cell ELISA was seen in patients with late LB.
Other commercially available immunoassays that contain mixtures of purified or recombinant antigens have been formatted as dot blot assays on strips. One of these assays uses whole borrelial lysates plus recombinant high-molecular-weight antigens, purified flagellin, recombinant flagellin, and recombinant OspC. Another uses whole borrelial lysates plus recombinant P39 and purified flagellin (6). Limited information is available on the performance of these assays.
Bacon et al. (15) evaluated recombinant VlsE for detection of IgG and/or IgM antibodies, the peptide pepC10 for detection of IgM antibodies, and the peptide C6 for the detection of IgG antibodies, in comparison to the two-tier approach, for testing sera from 280 patients with LB. They analyzed the results of individual assays and of their potential combinations. The overall best sensitivity was seen with the combination of C6 IgG and pepC10 IgM compared with two-tier testing (78% versus 68%, respectively). The greatest difference was observed with sera of patients with EM; 63% of samples were positive for C6 IgG-pepC10 IgM during the acute phase, compared with 38% by two-tier testing. By convalescence after treatment, 80% and 67% were positive with the combined peptides and two-tier testing, respectively. Both VlsE IgG-pepC10 IgM and VlsE IgG-VlsE IgM had higher sensitivity (100%) in sera of patients with early neuroborreliosis compared with C6 IgG-pepC10 IgM (73%) or two-tier testing (82%). All test combinations had high sensitivities in late LB. The specificity of these assays was set at ≥98%.
Whether the use of immunoassays using recombinant or synthetic peptide antigens will become the standard of practice for diagnosis of LB is currently unknown. More studies are needed to determine the applicability of such antigens to the serodiagnosis of patients with various manifestations of LB in both the United States and Europe. A desirable feature is that they are potentially amenable to automation, which would avoid the subjectivity of immunoblot interpretation. The cost will depend on patent and ownership issues.
Other antibody detection methods. (i) Functional antibodies: borreliacidal antibody assays.
Antibodies to certain antigens of B. burgdorferi sensu lato, in particular to certain outer surface proteins, may have bactericidal activity (46, 132, 249, 286, 287). Borreliacidal antibodies have been used in the immunodiagnosis of early and late LB by a few centers (5, 47, 48). In these assays live B. burgdorferi sensu stricto is incubated with patient serum plus an exogenous source of complement for 16 to 72 h (47). Growth inhibition of B. burgdorferi sensu stricto can be determined by visual inspection of the percentage of nonmotile spirochetes, color changes by use of a pH indicator, or flow cytometry after staining with acridine orange. The advantage of these assays is their high specificity in untreated patients compared with matrix-based nonfunctional assays. Invariably these tests are nonreactive in healthy control populations or in sera of non-Lyme disease patients with rheumatological diseases (47, 48). Modifications of the assay with reported higher sensitivities employ B. burgdorferi sensu stricto strain 50772, which lacks OspA and OspB (44, 45). Major disadvantages include the need for cultured live B. burgdorferi sensu stricto, interference from antimicrobials that might be present in patient sera, and the relatively cumbersome nature of the assays.
(ii) Detection of antibodies bound to circulating immune complexes.
It has been proposed that seronegativity in early LB is mainly due to the formation of specific antigen-antibody complexes that impede the detection of free antibodies by conventional methods. Some studies have suggested that IC are found not only in serum but also in other body fluids such as cerebrospinal and synovial fluids of patients with LB (68, 116, 295, 379).
Detection of antibodies bound to immune complexes involves the treatment of serum with polyethylene glycol to precipitate the IC, followed by dissociation through alkalinization of the complexes to release antibodies that can then be detected by ELISA or IB. A recent modification of this assay, the enzyme-linked IgM capture IC biotinylated antigen assay, was compared to IgG and IgM ELISA and IB with sera collected from clinically defined patients with LB and a control population. The enzyme-linked IgM capture IC biotinylated antigen assay was found to be more sensitive and specific than the aforementioned tests and furthermore detected antibodies more consistently in those patients with clinical evidence of active disease (62 of 64 patients; 97%) than in those with past infection (4 of 28; 14%). In that study, standard IgM ELISA and IgM IB were reactive in 67% and 58%, respectively, of patients with active disease and in 43% and 39%, respectively, of patients with past infection (36).
Potential utilities of this type of assay include detection of antibodies in seronegative patients during early disease and ascertainment of whether persistent seropositivity is due to ongoing infection, since IC are speculated to be present only in active infection (36). Although these assays appear to be helpful in certain clinical scenarios, only a few investigators have used them.
Detection of antibodies in cerebrospinal fluid.
There are currently no FDA-approved tests to measure intrathecal production of antibodies in CSF. Methods that have been used to detect antibodies in CSF include capture immunoassays, CSF/serum indices determined by ELISA, and Western immunoblots (69, 113, 364). Determination of intrathecal production of antibodies can be accomplished by measuring the CSF/serum index of B. burgdorferi sensu lato antibodies. CSF and serum samples diluted to match the total IgG concentration in CSF are run in parallel in an IgG ELISA. Positive intrathecal production is indicated by CSF/serum optical density ratios of >1.3 (364). Intrathecal production can also be determined by testing CSF and serum at matching concentrations of IgG and IgM in IgG and IgM IB, respectively. Greater numbers and intensities of bands in CSF compared with the corresponding serum would be consistent with intrathecal antibody production, but this has not been well substantiated (356). Specific intrathecal production of IgG, IgA, and/or IgM B. burgdorferi sensu lato antibodies has been described by several investigators (56, 58, 59, 141, 340). Recombinant antigens have also been studied in Europe for evaluation of intrathecal antibody production (142, 143, 245). The use of recombinant internal fragment of flagellin, although of lower sensitivity than whole-cell preparations, has shown greater specificity in the detection of B. burgdorferi sensu lato antibodies in CSF (142, 143). Detection of intrathecal antibodies by using three variants of the recombinant proteins DbpA, BBK32, and OspC, each variant originating from a different species of Lyme Borrelia, plus synthetic peptide IR6 as the source of antigens was superior to a commercial flagellum-based ELISA in a Finnish study of 89 patients with neuroborreliosis (245). In this study, the highest sensitivities for each antigen for the detection of IgG antibodies in CSF were 88% for DbpA, 80% for IR6, 76% for BBK32, 75% for OspC, and 52% for flagella.
Intrathecal B. burgdorferi sensu lato antibody detection in patients with neuroborreliosis in the United States seems to be less frequent than that in such patients from Europe. A factor contributing to this discrepancy may be the difference in B. burgdorferi sensu lato species causing neuroborreliosis in Europe and the United States. Several European studies dealing with typing of B. burgdorferi sensu lato strains cultured from CSF samples or detected by PCR have demonstrated that the most frequent species is B. garinii (42, 166, 285). Only four of 36 (11%) CSF isolates from German patients and 1 of 40 (2.5%) CSF isolates from Slovenian patients were B. burgdorferi sensu stricto (42, 285).
Cellular immune response in LB: T-lymphocyte and mononuclear cell proliferation assays.
The development of a cellular immune response in Lyme borreliosis has been observed in assays using peripheral blood mononuclear cells or cells present in affected tissues of patients with LB (37, 38, 73, 240, 305). However, the initial enthusiasm for use of an in vitro lymphoproliferation assay for diagnosis of LB has been tempered. T-lymphocyte proliferation assays have seldom been used as diagnostic tests due to their cumbersome nature and concerns about specificity and standardization (126).
TEST INTERPRETATION
Of the nonculture direct methods of detection, PCR is the most promising in providing assistance in the diagnosis of LB. A positive PCR result in a synovial fluid specimen of a patient with exposure to an area where LB is endemic and who also has a positive B. burgdorferi sensu lato ELISA and IgG IB is strongly supportive of a diagnosis of Lyme arthritis. Untreated patients with Lyme arthritis, as well as patients during the first days to weeks of therapy, may exhibit a positive PCR result in synovial fluid.
Positive PCR results for B. burgdorferi sensu lato nucleic acids obtained in samples from patients with possible extracutaneous manifestations of LB in the absence of serological evidence of B. burgdorferi sensu lato infection, however, should be interpreted with caution. In those situations, PCRs are often false positive. One of the limitations of nucleic acid amplification methods is the generation of false-positive results due to contamination. Adherence to rigorous quality control steps is of utmost importance.
Interpretation of serology in LB requires an understanding of the use and limitations of the currently available tests for B. burgdorferi sensu lato antibodies.
(i) These tests detect antibodies reacting with B. burgdorferi sensu lato and can support the clinical suspicion of LB, but in and of themselves they do not diagnose LB.
(ii) Antibodies may not be detectable at the time the patient presents with signs and symptoms of early LB with EM, and their presence correlates directly with the duration of disease prior to seeking medical attention, as well as with the presence of symptoms or objective signs of dissemination (for example, multiple EMs or cranial nerve palsies). For patients with early LB who have EM, the diagnosis is established on clinical grounds if the skin lesion is characteristic. In situations where the skin lesion is atypical or absent, tests to detect B. burgdorferi sensu lato antibodies may be necessary, as follows.
(a) When antibodies are not detectable or not diagnostic in the acute-phase serum specimen, a convalescent-phase specimen should be collected 2 to 4 weeks later and tested for B. burgdorferi sensu lato antibodies (7, 89).
(b) In patients treated with antimicrobials, testing of the convalescent-phase sample within 1 month of onset of symptoms will maximize sensitivity, since the criteria for test positivity at this time point include IgM IB seroconversion, whereas after 1 month IgG seroconversion is required for seropositivity.
(iii) Testing for B. burgdorferi sensu lato antibodies should include the two-tier approach as currently recommended (53). In those specimens testing positive or equivocal by first-tier assays, second-tier IB is used to improve specificity. IB should not be used with sera testing negative by the first tier, as this would reduce specificity compared to the two-tier testing strategy (369a). Patients who received the OspA vaccine may be seropositive due to the presence of OspA in whole-cell B. burgdorferi sensu lato preparations or in other antigen preparations that include recombinant OspA in the mixture. Vaccine reactivity can often be distinguished from antibodies elicited by natural infection with B. burgdorferi sensu stricto by IB (9) (Fig. 1, right).
(iv) B. burgdorferi sensu lato antibodies, both IgG and IgM, may persist for many years after successful treatment of LB (93). Thus, persistent seropositivity is not, per se, an indication of treatment failure. Persistence of antibodies to B. burgdorferi sensu lato in sera of individuals residing in areas where LB is endemic and who have been treated for LB, or who have resolved an asymptomatic infection, may limit the utility of future serologic testing as a diagnostic tool when such persons present with a new clinical event. In these circumstances, a change in reactivity between acute- and convalescent-phase specimens may be of assistance, although this has never been systematically evaluated. An increase in antibody concentration as determined by optical density in EIA, or by IFA titers, in first-tier assays or by an increase in intensity or appearance of new immunoreactive bands by IB might suggest a new or recent B. burgdorferi sensu lato infection. Physicians caring for these patients should store an aliquot of an acute-phase serum specimen to be submitted along with the convalescent-phase sample for testing in parallel for B. burgdorferi sensu lato antibodies.
(v) Patients with late manifestations of LB usually have a high concentration of antibodies by first-step tests and have numerous immunoreactive bands in IgG blots, often far surpassing the number of bands required in the IgG IB interpretation criteria (84). A lack of seropositivity in patients suspected of having late LB practically excludes this diagnosis.
(vi) Laboratories performing B. burgdorferi sensu lato serology should use assays of proven performance as determined by the summary of surveys run by the College of American Pathologists or local or state proficiency programs.
(vii) Laboratorians should make an attempt to avoid scoring weak bands leading to false-positive readings in IB, particularly IgM IB. This can be avoided by strict adherence to comparison of band intensity to those of cutoff intensity control materials.
(viii) The use of laboratory tests or interpretation strategies that have not been appropriately validated is of great concern and is strongly discouraged (53). Currently there are commercial laboratories offering B. burgdorferi sensu lato urine antigen tests, immunofluorescent staining for cell wall-deficient forms of B. burgdorferi sensu lato, and lymphocyte transformation tests. In addition, some laboratories are performing PCR for B. burgdorferi sensu lato DNA on inappropriate samples such as blood and urine or are interpreting IB by using criteria that have not been validated (53).
(ix) The use of B. burgdorferi sensu lato antibody testing should be restricted to those patients with a 0.2 to 0.8 pretest probability of having LB as recommended by the American College of Physicians (339). The use of these tests in unselected populations with a low pretest probability of the disease is more likely to yield false-positive than true-positive results. Testing is not recommended for patients presenting with classic EM, since treatment without testing is more cost-effective and B. burgdorferi sensu lato antibody assays have low sensitivity at this stage (222). Since the specificity of B. burgdorferi sensu lato antibody testing is not 100% and since approximately 2.7 million tests are estimated to be done yearly in the United States, for every 1% reduction in test specificity there will be approximately 27,000 false-positive results per year, dwarfing the true positive incidence of about 20,000 cases/year.
Acknowledgments
We thank Charles Pavia, Susan Bittker, Denise Cooper, Carol Carbonaro, Janet Roberge, and Lois Zentmaier for technical assistance and Lisa Giarratano for secretarial help.
REFERENCES
- 1.Aberer, E., C. Brunner, G. Suchanek, H. Klade, A. G. Barbour, G. Stanek, and H. Lassmann. 1989. Molecular mimicry and Lyme borreliosis: a shared antigenic determinant between Borrelia burgdorferi and human tissue. Ann. Neurol. 26:732-737. [DOI] [PubMed] [Google Scholar]
- 2.Abu Al-Soud, W., and P. Radstrom. 2001. Purification and characterization of PCR-inhibitory components in blood cells. J. Clin. Microbiol. 39:485-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann, R., J. Kabatzki, H. P. Boisten, A. C. Steere, R. L. Grodzicki, S. Hartung, and U. Runne. 1984. Spirochete etiology of erythema chronicum migrans disease. Dtsch. Med. Wochenschr. 109:92-97. [DOI] [PubMed] [Google Scholar]
- 4.Agger, W. A., S. M. Callister, and D. A. Jobe. 1992. In vitro susceptibilities of Borrelia burgdorferi to five oral cephalosporins and ceftriaxone. Antimicrob. Agents Chemother. 36:1788-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agger, W. A., and K. L. Case. 1997. Clinical comparison of borreliacidal-antibody test with indirect immunofluorescence and enzyme-linked immunosorbent assays for diagnosis of Lyme disease. Mayo Clin. Proc. 72:510-514. [DOI] [PubMed] [Google Scholar]
- 6.Aguero-Rosenfeld, M. E. 2004. Detection of Borrelia burgdorferi antibodies, p. 11.6.1-11.6.10. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook, 2nd ed. ASM Press, Washington, D.C.
- 7.Aguero-Rosenfeld, M. E., J. Nowakowski, S. Bittker, D. Cooper, R. B. Nadelman, and G. P. Wormser. 1996. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J. Clin. Microbiol. 34:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguero-Rosenfeld, M. E., J. Nowakowski, D. F. McKenna, C. A. Carbonaro, and G. P. Wormser. 1993. Serodiagnosis in early Lyme disease. J. Clin. Microbiol. 31:3090-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguero-Rosenfeld, M. E., J. Roberge, C. A. Carbonaro, J. Nowakowski, R. B. Nadelman, and G. P. Wormser. 1999. Effects of OspA vaccination on Lyme disease serologic testing. J. Clin. Microbiol. 37:3718-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akin, E., G. L. McHugh, R. A. Flavell, E. Fikrig, and A. C. Steere. 1999. The immunoglobulin G (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect. Immun. 67:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amouriaux, P., M. Assous, D. Margarita, G. Baranton, and I. Saint Girons. 1993. Polymerase chain reaction with the 30-kb circular plasmid of Borrelia burgdorferi B31 as a target for detection of the Lyme borreliosis agents in cerebrospinal fluid. Res. Microbiol. 144:211-219. [DOI] [PubMed] [Google Scholar]
- 12.Anderson, J. F. 1991. Epizootiology of Lyme borreliosis. Scand. J. Infect. Dis. Suppl. 77:23-34. [PubMed] [Google Scholar]
- 13.Arnez, M., E. Ruzic-Sabljic, J. Ahcan, A. Radsel-Medvescek, D. Pleterski-Rigler, and F. Strle. 2001. Isolation of Borrelia burgdorferi sensu lato from blood of children with solitary erythema migrans. Pediatr. Infect. Dis. J. 20:251-255. [DOI] [PubMed] [Google Scholar]
- 14.Asbrink, E., and A. Hovmark. 1985. Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathol. Microbiol. Immunol. Scand. B 93:161-163. [DOI] [PubMed] [Google Scholar]
- 15.Bacon, R. M., B. J. Biggerstaff, M. E. Schriefer, R. D. Gilmore, Jr., M. T. Philipp, A. C. Steere, G. P. Wormser, A. R. Marques, and B. J. Johnson. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 187:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baranton, G., D. Postic, I. Saint Girons, P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 17.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 18.Barbour, A. G. 1988. Laboratory aspects of Lyme borreliosis. Clin. Microbiol. Rev. 1:399-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbour, A. G., W. Burgdorfer, S. F. Hayes, O. Peter, and A. Aeschilimann. 1983. Isolation of a cultivable spirochete from Ixodes ricinus ticks of Switzerland. Curr. Microbiol. 8:123-126. [Google Scholar]
- 20.Barbour, A. G., and S. F. Hayes. 1986. Biology of Borrelia species. Microbiol. Rev. 50:381-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 22.Benach, J. L., E. M. Bosler, J. P. Hanrahan, J. L. Coleman, G. S. Habicht, T. F. Bast, D. J. Cameron, J. L. Ziegler, A. G. Barbour, W. Burgdorfer, R. Edelman, and R. A. Kaslow. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308:740-742. [DOI] [PubMed] [Google Scholar]
- 23.Berger, B. W. 1989. Dermatologic manifestations of Lyme disease. Rev. Infect. Dis. 11(Suppl. 6):S1475-S1481. [DOI] [PubMed] [Google Scholar]
- 24.Berger, B. W., O. J. Clemmensen, and A. B. Ackerman. 1983. Lyme disease is a spirochetosis. A review of the disease and evidence for its cause. Am. J. Dermatopathol. 5:111-124. [PubMed] [Google Scholar]
- 25.Berger, B. W., R. C. Johnson, C. Kodner, and L. Coleman. 1992. Cultivation of Borrelia burgdorferi from erythema migrans lesions and perilesional skin. J. Clin. Microbiol. 30:359-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger, B. W., R. C. Johnson, C. Kodner, and L. Coleman. 1992. Failure of Borrelia burgdorferi to survive in the skin of patients with antibiotic-treated Lyme disease. J. Am. Acad. Dermatol. 27:34-37. [DOI] [PubMed] [Google Scholar]
- 27.Bergler-Klein, J., H. Sochor, G. Stanek, S. Globits, R. Ullrich, and D. Glogar. 1993. Indium 111-monoclonal antimyosin antibody and magnetic resonance imaging in the diagnosis of acute Lyme myopericarditis. Arch. Intern. Med. 153:2696-2700. [PubMed] [Google Scholar]
- 28.Bergmann, A. R., B. L. Schmidt, A. M. Derler, and E. Aberer. 2002. Importance of sample preparation for molecular diagnosis of Lyme borreliosis from urine. J. Clin. Microbiol. 40:4581-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergstrom, S., A. G. Barbour, C. F. Garon, P. Hindersson, I. Saint Girons, I. Girons, and T. G. Schwan. 1991. Genetics of Borrelia burgdorferi. Scand. J. Infect. Dis. Suppl. 77:102-107. [PubMed] [Google Scholar]
- 30.Bradley, J. F., R. C. Johnson, and J. L. Goodman. 1994. The persistence of spirochetal nucleic acids in active Lyme arthritis. Ann. Intern. Med. 120:487-489. [DOI] [PubMed] [Google Scholar]
- 31.Brettschneider, S., H. Bruckbauer, N. Klugbauer, and H. Hofmann. 1998. Diagnostic value of PCR for detection of Borrelia burgdorferi in skin biopsy and urine samples from patients with skin borreliosis. J. Clin. Microbiol. 36:2658-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown, E. L., R. M. Wooten, B. J. Johnson, R. V. Iozzo, A. Smith, M. C. Dolan, B. P. Guo, J. J. Weis, and M. Hook. 2001. Resistance to Lyme disease in decorin-deficient mice. J. Clin. Investig. 107:845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown, S. L., S. L. Hansen, and J. J. Langone. 1999. Role of serology in the diagnosis of Lyme disease. JAMA 282:62-66. [DOI] [PubMed] [Google Scholar]
- 35.Bruckbauer, H. R., V. Preac-Mursic, R. Fuchs, and B. Wilske. 1992. Cross-reactive proteins of Borrelia burgdorferi. Eur. J. Clin. Microbiol. Infect. Dis. 11:224-232. [DOI] [PubMed] [Google Scholar]
- 36.Brunner, M., and L. H. Sigal. 2001. Use of serum immune complexes in a new test that accurately confirms early Lyme disease and active infection with Borrelia burgdorferi. J. Clin. Microbiol. 39:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buechner, S. A., S. Lautenschlager, P. Itin, A. Bircher, and P. Erb. 1995. Lymphoproliferative responses to Borrelia burgdorferi in patients with erythema migrans, acrodermatitis chronica atrophicans, lymphadenosis benigna cutis, and morphea. Arch. Dermatol. 131:673-677. [PubMed] [Google Scholar]
- 38.Buechner, S. A., T. Rufli, and P. Erb. 1993. Acrodermatitis chronic atrophicans: a chronic T-cell-mediated immune reaction against Borrelia burgdorferi? Clinical, histologic, and immunohistochemical study of five cases. J. Am. Acad. Dermatol. 28:399-405. [DOI] [PubMed] [Google Scholar]
- 39.Bunikis, J., and A. G. Barbour. 2002. Laboratory testing for suspected Lyme disease. Med. Clin. North Am. 86:311-340. [DOI] [PubMed] [Google Scholar]
- 40.Burgdorfer, W., J. F. Anderson, L. Gern, R. S. Lane, J. Piesman, and A. Spielman. 1991. Relationship of Borrelia burgdorferi to its arthropod vectors. Scand. J. Infect. Dis. Suppl. 77:35-40. [PubMed] [Google Scholar]
- 41.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 42.Busch, U., C. Hizo-Teufel, R. Boehmer, V. Fingerle, H. Nitschko, B. Wilske, and V. Preac-Mursic. 1996. Three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B afzelii, and B. garinii) identified from cerebrospinal fluid isolates by pulsed-field gel electrophoresis and PCR. J. Clin. Microbiol. 34:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callister, S. M., K. L. Case, W. A. Agger, R. F. Schell, R. C. Johnson, and J. L. Ellingson. 1990. Effects of bovine serum albumin on the ability of Barbour-Stoenner-Kelly medium to detect Borrelia burgdorferi. J. Clin. Microbiol. 28:363-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Callister, S. M., D. A. Jobe, W. A. Agger, R. F. Schell, T. J. Kowalski, S. D. Lovrich, and J. A. Marks. 2002. Ability of the borreliacidal antibody test to confirm Lyme disease in clinical practice. Clin. Diagn. Lab. Immunol. 9:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callister, S. M., D. A. Jobe, R. F. Schell, C. S. Pavia, and S. D. Lovrich. 1996. Sensitivity and specificity of the borreliacidal-antibody test during early Lyme disease: a “gold standard”? Clin. Diagn. Lab. Immunol. 3:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callister, S. M., R. F. Schell, K. L. Case, S. D. Lovrich, and S. P. Day. 1993. Characterization of the borreliacidal antibody response to Borrelia burgdorferi in humans: a serodiagnostic test. J. Infect. Dis. 167:158-164. [DOI] [PubMed] [Google Scholar]
- 47.Callister, S. M., R. F. Schell, L. C. Lim, D. A. Jobe, K. L. Case, G. L. Bryant, and P. E. Molling. 1994. Detection of borreliacidal antibodies by flow cytometry. An accurate, highly specific serodiagnostic test for Lyme disease. Arch. Intern. Med. 154:1625-1632. [PubMed] [Google Scholar]
- 48.Callister, S. M., R. F. Schell, and S. D. Lovrich. 1991. Lyme disease assay which detects killed Borrelia burgdorferi. J. Clin. Microbiol. 29:1773-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canica, M. M., F. Nato, L. du Merle, J. C. Mazie, G. Baranton, and D. Postic. 1993. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand. J. Infect. Dis. 25:441-448. [DOI] [PubMed] [Google Scholar]
- 50.Casjens, S. 2000. Borrelia genomes in the year 2000. J. Mol. Microbiol. Biotechnol. 2:401-410. [PubMed] [Google Scholar]
- 51.Casjens, S., M. Delange, H. L. Ley III, P. Rosa, and W. M. Huang. 1995. Linear chromosomes of Lyme disease agent spirochetes: genetic diversity and conservation of gene order. J. Bacteriol. 177:2769-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casjens, S., N. Palmer, R. van Vugt, H. W. Mun, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. 2004. Lyme disease—United States, 2001-2002. Morb. Mortal. Wkly. Rep. 53:365-369. [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. 2005. Notice to readers: caution regarding testing for Lyme disease. Morb. Mortal. Wkly. Rep. 54:125. [Google Scholar]
- 56.Christen, H. J., N. Bartlau, F. Hanefeld, H. Eiffert, and R. Thomssen. 1990. Peripheral facial palsy in childhood—Lyme borreliosis to be suspected unless proven otherwise. Acta Paediatr. Scand. 79:1219-1224. [DOI] [PubMed] [Google Scholar]
- 57.Christen, H. J., H. Eiffert, A. Ohlenbusch, and F. Hanefeld. 1995. Evaluation of the polymerase chain reaction for the detection of Borrelia burgdorferi in cerebrospinal fluid of children with acute peripheral facial palsy. Eur. J. Pediatr. 154:374-377. [DOI] [PubMed] [Google Scholar]
- 58.Christen, H. J., F. Hanefeld, H. Eiffert, and R. Thomssen. 1993. Epidemiology and clinical manifestations of Lyme borreliosis in childhood. A prospective multicentre study with special regard to neuroborreliosis. Acta Paediatr. Suppl. 386:1-75. [DOI] [PubMed] [Google Scholar]
- 59.Christova, I., E. Tasseva, B. Stamenov, and K. Sabev. 2000. Neuroborreliosis in Bulgaria: detection of Borrelia burgdorferi-specific intrathecal antibody production. Int. J. Immunopathol. Pharmacol. 13:99-106. [PubMed] [Google Scholar]
- 60.Cimperman, J., V. Maraspin, S. Lotric-Furlan, E. Ruzic-Sabljic, and F. Strle. 1999. Lyme meningitis: a one-year follow up controlled study. Wien. Klin. Wochenschr. 111:961-963. [PubMed] [Google Scholar]
- 61.Cinco, M., M. Ruscio, and F. Rapagna. 2000. Evidence of Dbps (decorin binding proteins) among European strains of Borrelia burgdorferi sensu lato and in the immune response of LB patient sera. FEMS Microbiol Lett. 183:111-114. [DOI] [PubMed] [Google Scholar]
- 62.Cogswell, F. B., C. E. Bantar, T. G. Hughes, Y. Gu, and M. T. Philipp. 1996. Host DNA can interfere with detection of Borrelia burgdorferi in skin biopsy specimens by PCR. J. Clin. Microbiol. 34:980-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coleman, J. L., and J. L. Benach. 1987. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J. Infect. Dis. 155:756-765. [DOI] [PubMed] [Google Scholar]
- 64.Coleman, J. L., and J. L. Benach. 1992. Characterization of antigenic determinants of Borrelia burgdorferi shared by other bacteria. J. Infect. Dis. 165:658-666. [DOI] [PubMed] [Google Scholar]
- 65.Collares-Pereira, M., S. Couceiro, I. Franca, K. Kurtenbach, S. M. Schafer, L. Vitorino, L. Goncalves, S. Baptista, M. L. Vieira, and C. Cunha. 2004. First isolation of Borrelia lusitaniae from a human patient. J. Clin. Microbiol. 42:1316-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Courtney, J. W., and R. F. Massung. 2003. Multiplex Taqman PCR assay for rapid detection of Anaplasma phagocytophila and Borrelia burgdorferi. Ann. N. Y. Acad. Sci. 990:369-370. [DOI] [PubMed] [Google Scholar]
- 67.Coyle, P. K., Z. Deng, S. E. Schutzer, A. L. Belman, J. Benach, L. B. Krupp, and B. Luft. 1993. Detection of Borrelia burgdorferi antigens in cerebrospinal fluid. Neurology 43:1093-1098. [DOI] [PubMed] [Google Scholar]
- 68.Coyle, P. K., S. E. Schutzer, A. L. Belman, L. B. Krupp, and M. G. Golightly. 1990. Cerebrospinal fluid immune complexes in patients exposed to Borrelia burgdorferi: detection of Borrelia-specific and -nonspecific complexes. Ann. Neurol. 28:739-744. [DOI] [PubMed] [Google Scholar]
- 69.Coyle, P. K., S. E. Schutzer, Z. Deng, L. B. Krupp, A. L. Belman, J. L. Benach, and B. J. Luft. 1995. Detection of Borrelia burgdorferi-specific antigen in antibody-negative cerebrospinal fluid in neurologic Lyme disease. Neurology 45:2010-2015. [DOI] [PubMed] [Google Scholar]
- 70.Craft, J. E., D. K. Fischer, G. T. Shimamoto, and A. C. Steere. 1986. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J. Clin. Investig. 78:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crother, T. R., C. I. Champion, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2003. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect. Immun. 71:3419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Das, S., S. W. Barthold, S. S. Giles, R. R. Montgomery, S. R. 3. Telford, E. Fikrig, and D. H. Persing. 1997. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J. Clin. Investig. 99:987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dattwyler, R. J., D. J. Volkman, B. J. Luft, J. J. Halperin, J. Thomas, and M. G. Golightly. 1988. Seronegative Lyme disease. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N. Engl. J. Med. 319:1441-1446. [DOI] [PubMed] [Google Scholar]
- 74.Debue, M., P. Gautier, C. Hackel, A. Van Elsen, A. Herzog, and G. X. B. A. Bigaignon. 1991. Detection of Borrelia burgdorferi in biological samples using the polymerase chain reaction assay. Res. Microbiol. 142:565-572. [DOI] [PubMed] [Google Scholar]
- 75.de Koning, J., R. B. Bosma, and J. A. Hoogkamp-Korstanje. 1987. Demonstration of spirochaetes in patients with Lyme disease with a modified silver stain. J. Med. Microbiol. 23:261-267. [DOI] [PubMed] [Google Scholar]
- 76.de Koning, J., and P. H. Duray. 1993. Histopathology of human Lyme borreliosis, p. 70-92. In K. Weber and W. Burgdorfer (ed.), Aspects of Lyme borreliosis. Springer-Verlag, Berlin, Germany.
- 77.de Koning, J., J. A. Hoogkamp-Korstanje, M. R. van der Linde, and H. J. Crijns. 1989. Demonstration of spirochetes in cardiac biopsies of patients with Lyme disease. J. Infect. Dis. 160:150-153. [DOI] [PubMed] [Google Scholar]
- 78.Demaerschalck, I., A. Ben Messaoud, M. De Kesel, B. Hoyois, Y. Lobet, P. P. Hoet, G. Bigaignon, A. Bollen, and E. Godfroid. 1995. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J. Clin. Microbiol. 33:602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Silva, A. M., and E. Fikrig. 1997. Borrelia burgdorferi genes selectively expressed in ticks and mammals. Parasitol. Today 13:267-270. [DOI] [PubMed] [Google Scholar]
- 80.de Silva, A. M., E. Fikrig, E. Hodzic, F. S. Kantor, S. R. Telford, and S. W. Barthold. 1998. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J. Infect. Dis. 177:395-400. [DOI] [PubMed] [Google Scholar]
- 81.Diza, E., A. Papa, E. Vezyri, S. Tsounis, I. Milonas, and A. Antoniadis. 2004. Borrelia valaisiana in cerebrospinal fluid. Emerg. Infect. Dis. 10:1692-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dorn, G. L., G. G. Burson, and J. R. Haynes. 1976. Blood culture technique based on centrifugation: clinical evaluation. J. Clin. Microbiol. 3:258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dorward, D. W., T. G. Schwan, and C. F. Garon. 1991. Immune capture and detection of Borrelia burgdorferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. J. Clin. Microbiol. 29:1162-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dressler, F., J. A. Whalen, B. N. Reinhardt, and A. C. Steere. 1993. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 167:392-400. [DOI] [PubMed] [Google Scholar]
- 85.Dumler, J. S. 2001. Molecular diagnosis of Lyme disease: Review and meta-analysis. Mol. Diagn. 6:1-11. [DOI] [PubMed] [Google Scholar]
- 86.Dykhuizen, D. E., and G. Baranton. 2001. Borrelia burgdorferi: a (somewhat) clonal bacterial species. Trends Microbiol. 9:472. [DOI] [PubMed] [Google Scholar]
- 87.Eiffert, H., A. Karsten, R. Thomssen, and H. J. Christen. 1998. Characterization of Borrelia burgdorferi strains in Lyme arthritis. Scand. J. Infect. Dis. 30:265-268. [DOI] [PubMed] [Google Scholar]
- 88.Eiffert, H., A. Ohlenbusch, H. J. Christen, R. Thomssen, A. Spielman, and F. R. Matuschka. 1995. Nondifferentiation between Lyme disease spirochetes from vector ticks and human cerebrospinal fluid. J. Infect. Dis. 171:476-479. [DOI] [PubMed] [Google Scholar]
- 89.Engstrom, S. M., E. Shoop, and R. C. Johnson. 1995. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 33:419-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Exner, M. M., and M. A. Lewinski. 2003. Isolation and detection of Borrelia burgdorferi DNA from cerebral spinal fluid, synovial fluid, blood, urine, and ticks using the Roche MagNA Pure system and real-time PCR. Diagn. Microbiol. Infect. Dis. 46:235-240. [DOI] [PubMed] [Google Scholar]
- 91.Fawcett, P. T., K. M. Gibney, C. D. Rose, S. B. Dubbs, and R. A. Doughty. 1992. Frequency and specificity of antibodies that crossreact with Borrelia burgdorferi antigens. J. Rheumatol. 19:582-587. [PubMed] [Google Scholar]
- 92.Fawcett, P. T., C. Rose, K. M. Gibney, C. A. Chase, B. Kiehl, and R. A. Doughty. 1993. Detection of antibodies to the recombinant P39 protein of Borrelia burgdorferi using enzyme immunoassay and immunoblotting. J. Rheumatol. 20:734-738. [PubMed] [Google Scholar]
- 93.Feder, H. M., Jr., M. A. Gerber, S. W. Luger, and R. W. Ryan. 1992. Persistence of serum antibodies to Borrelia burgdorferi in patients treated for Lyme disease. Clin. Infect. Dis. 15:788-793. [DOI] [PubMed] [Google Scholar]
- 94.Fikrig, E., S. W. Barthold, W. Sun, W. Feng, S. R. Telford, and R. A. Flavell. 1997. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity 6:531-539. [DOI] [PubMed] [Google Scholar]
- 95.Fikrig, E., W. Feng, J. Aversa, R. T. Schoen, and R. A. Flavell. 1998. Differential expression of Borrelia burgdorferi genes during erythema migrans and Lyme arthritis. J. Infect. Dis. 178:1198-1201. [DOI] [PubMed] [Google Scholar]
- 96.Fikrig, E., W. Feng, S. W. Barthold, S. R. Telford III, and R. A. Flavell. 2000. Arthropod- and host-specific Borrelia burgdorferi bbk32 expression and the inhibition of spirochete transmission. J. Immunol. 164:5344-5351. [DOI] [PubMed] [Google Scholar]
- 97.Fikrig, E., E. D. Huguenel, R. Berland, D. W. Rahn, J. A. Hardin, and R. A. Flavell. 1992. Serologic diagnosis of Lyme disease using recombinant outer surface proteins A and B and flagellin. J. Infect. Dis. 165:1127-1132. [DOI] [PubMed] [Google Scholar]
- 98.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 99.Fukunaga, M., A. Hamase, K. Okada, and M. Nakao. 1996. Borrelia tanukii sp. nov. and Borrelia turdae sp. nov. found from ixodid ticks in Japan: rapid species identification by 16S rRNA gene-targeted PCR analysis. Microbiol. Immunol. 40:877-881. [DOI] [PubMed] [Google Scholar]
- 100.Fung, B. P., G. L. McHugh, J. M. Leong, and A. C. Steere. 1994. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect. Immun. 62:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gassmann, G. S., E. Jacobs, R. Deutzmann, and U. B. Gobel. 1991. Analysis of the Borrelia burgdorferi GeHo fla gene and antigenic characterization of its gene product. J. Bacteriol. 173:1452-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gerber, M. A., E. D. Shapiro, G. L. Bell, A. Sampieri, and S. J. Padula. 1995. Recombinant outer surface protein C ELISA for the diagnosis of early Lyme disease. J. Infect. Dis. 171:724-727. [DOI] [PubMed] [Google Scholar]
- 103.Gilmore, R. D., Jr., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 104.Gilmore, R. D. J., R. L. Murphree, A. M. James, S. A. Sullivan, and B. J. Johnson. 1999. The Borrelia burgdorferi 37-kilodalton immunoblot band (P37) used in serodiagnosis of early Lyme disease is the flaA gene product. J. Clin. Microbiol. 37:548-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glockner, G., R. Lehmann, A. Romualdi, S. Pradella, U. Schulte-Spechtel, M. Schilhabel, B. Wilske, J. Suhnel, and M. Platzer. 2004. Comparative analysis of the Borrelia garinii genome. Nucleic Acids Res. 32:6038-6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Godfroid, E., H. C. Min, P. F. Humair, A. Bollen, and L. Gern. 2003. PCR-reverse line blot typing method underscores the genomic heterogeneity of Borrelia valaisiana species and suggests its potential involvement in Lyme disease. J. Clin. Microbiol. 41:3690-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gomes-Solecki, M. J., J. J. Dunn, B. J. Luft, J. Castillo, D. E. Dykhuizen, X. Yang, J. D. Glass, and R. J. Dattwyler. 2000. Recombinant chimeric Borrelia proteins for diagnosis of Lyme disease. J. Clin. Microbiol. 38:2530-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goodman, J. L., J. F. Bradley, A. E. Ross, P. Goellner, A. Lagus, B. Vitale, B. W. Berger, S. Luger, and R. C. Johnson. 1995. Bloodstream invasion in early Lyme disease: results from a prospective, controlled, blinded study using the polymerase chain reaction. Am. J. Med. 99:6-12. [DOI] [PubMed] [Google Scholar]
- 109.Goodman, J. L., P. Jurkovich, J. M. Kramber, and R. C. Johnson. 1991. Molecular detection of persistent Borrelia burgdorferi in the urine of patients with active Lyme disease. Infect. Immun. 59:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Greene, R. T., R. L. Walker, and C. E. Greene. 1991. Pseudospirochetes in animal blood being cultured for Borrelia burgdorferi. J. Vet. Diagn. Investig. 3:350-352. [DOI] [PubMed] [Google Scholar]
- 111.Grodzicki, R. L., and A. C. Steere. 1988. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J. Infect. Dis. 157:790-797. [DOI] [PubMed] [Google Scholar]
- 112.Gross, D. M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z. A. Nagy, J. A. Field, A. C. Steere, and B. T. Huber. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281:703-706. [DOI] [PubMed] [Google Scholar]
- 113.Halperin, J. J., D. J. Volkman, and P. Wu. 1991. Central nervous system abnormalities in Lyme neuroborreliosis. Neurology 41:1571-1582. [DOI] [PubMed] [Google Scholar]
- 114.Hansen, K., and E. Asbrink. 1989. Serodiagnosis of erythema migrans and acrodermatitis chronica atrophicans by the Borrelia burgdorferi flagellum enzyme-linked immunosorbent assay. J. Clin. Microbiol. 27:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hansen, K., P. Hindersson, and N. S. Pedersen. 1988. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J. Clin. Microbiol. 26:338-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hardin, J. A., A. C. Steere, and S. E. Malawista. 1979. Immune complexes and the evolution of Lyme arthritis. Dissemination and localization of abnormal C1q binding activity. N. Engl. J. Med. 301:1358-1363. [DOI] [PubMed] [Google Scholar]
- 117.Hauser, U., H. Krahl, H. Peters, V. Fingerle, and B. Wilske. 1998. Impact of strain heterogeneity on Lyme disease serology in Europe: comparison of enzyme-linked immunosorbent assays using different species of Borrelia burgdorferi sensu lato. J. Clin. Microbiol. 36:427-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hauser, U., G. Lehnert, R. Lobentanzer, and B. Wilske. 1997. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J. Clin. Microbiol. 35:1433-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hauser, U., and B. Wilske. 1997. Enzyme-linked immunosorbent assays with recombinant internal flagellin fragments derived from different species of Borrelia burgdorferi sensu lato for the serodiagnosis of Lyme neuroborreliosis. Med. Microbiol. Immunol. (Berlin) 186:145-151. [DOI] [PubMed] [Google Scholar]
- 120.Hayes, S. F., and W. Burgdorfer. 1993. Ultrastructure of Borrelia burgdorferi, p. 29-43. In K. Weber and W. Burgdorfer (ed.), Aspects of Lyme borreliosis. Springer-Verlag, Berlin, Germany.
- 121.Hechemy, K. E., W. A. Samsonoff, H. L. Harris, and M. McKee. 1992. Adherence and entry of Borrelia burgdorferi in Vero cells. J. Med. Microbiol. 36:229-238. [DOI] [PubMed] [Google Scholar]
- 122.Heikkila, T., H. I. Huppertz, I. Seppala, H. Sillanpaa, H. Saxen, and P. Lahdenne. 2003. Recombinant or peptide antigens in the serology of Lyme arthritis in children. J. Infect. Dis. 187:1888-1894. [DOI] [PubMed] [Google Scholar]
- 123.Heikkila, T., I. Seppala, H. Saxen, J. Panelius, M. Peltomaa, T. Julin, S. A. Carlsson, and P. Lahdenne. 2002. Recombinant BBK32 protein in serodiagnosis of early and late Lyme borreliosis. J. Clin. Microbiol. 40:1174-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heikkila, T., I. Seppala, H. Saxen, J. Panelius, H. Yrjanainen, and P. Lahdenne. 2002. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J. Clin. Microbiol. 40:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoffmann, J. C., D. O. Stichtenoth, H. Zeidler, M. Follmann, A. Brandis, G. Stanek, and J. Wollenhaupt. 1995. Lyme disease in a 74-year-old forest owner with symptoms of dermatomyositis. Arthritis Rheum. 38:1157-1160. [DOI] [PubMed] [Google Scholar]
- 126.Horowitz, H. W., C. S. Pavia, S. Bittker, G. Forseter, D. Cooper, R. B. Nadelman, D. Byrne, R. C. Johnson, and G. P. Wormser. 1994. Sustained cellular immune responses to Borrelia burgdorferi: lack of correlation with clinical presentation and serology. Clin. Diagn. Lab. Immunol. 1:373-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu, L. T., and M. S. Klempner. 1997. Host-pathogen interactions in the immunopathogenesis of Lyme disease. J. Clin. Immunol. 17:354-365. [DOI] [PubMed] [Google Scholar]
- 128.Hubalek, Z., and J. Halouzka. 1997. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur. J. Epidemiol. 13:951-957. [DOI] [PubMed] [Google Scholar]
- 129.Hubner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huppertz, H. I., H. Schmidt, and H. Karch. 1993. Detection of Borrelia burgdorferi by nested polymerase chain reaction in cerebrospinal fluid and urine of children with neuroborreliosis. Eur. J. Pediatr. 152:414-417. [DOI] [PubMed] [Google Scholar]
- 131.Hyde, F. W., R. C. Johnson, T. J. White, and C. E. Shelburne. 1989. Detection of antigens in urine of mice and humans infected with Borrelia burgdorferi, etiologic agent of Lyme disease. J. Clin. Microbiol. 27:58-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ikushima, M., K. Matsui, F. Yamada, S. Kawahashi, and S. K. Nishikawa. 2000. Specific immune response to a synthetic peptide derived from outer surface protein C of Borrelia burgdorferi predicts protective borreliacidal antibodies. FEMS Immunol. Med. Microbiol. 29:15-21. [DOI] [PubMed] [Google Scholar]
- 133.Jaulhac, B., I. Chary-Valckenaere, J. Sibilia, R. M. Javier, Y. Piemont, J. L. Kuntz, H. Monteil, and J. Pourel. 1996. Detection of Borrelia burgdorferi by DNA amplification in synovial tissue samples from patients with Lyme arthritis. Arthritis Rheum. 39:736-745. [DOI] [PubMed] [Google Scholar]
- 134.Jaulhac, B., P. Nicolini, Y. Piemont, and H. Monteil. 1991. Detection of Borrelia burgdorferi in cerebrospinal fluid of patients with Lyme borreliosis. N. Engl. J. Med. 324:1440. [PubMed] [Google Scholar]
- 135.Jauris-Heipke, S., R. Fuchs, M. Motz, V. Preac-Mursic, E. Schwab, E. Soutschek, G. Will, and B. Wilske. 1993. Genetic heterogenity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med. Microbiol. Immunol. (Berlin) 182:37-50. [DOI] [PubMed] [Google Scholar]
- 136.Jauris-Heipke, S., B. Rossle, G. Wanner, C. Habermann, D. Rossler, V. Fingerle, G. Lehnert, R. Lobentanzer, I. Pradel, B. Hillenbrand, U. Schulte-Spechtel, and B. Wilske. 1999. Osp17, a novel immunodominant outer surface protein of Borrelia afzelii: recombinant expression in Escherichia coli and its use as a diagnostic antigen for serodiagnosis of Lyme borreliosis. Med. Microbiol. Immunol. (Berlin) 187:213-219. [DOI] [PubMed] [Google Scholar]
- 137.Johnson, B. J., B. J. Biggerstaff, R. M. Bacon, and M. E. Schriefer. 2004. Cost-effectiveness of peptide-antigen immunoassays for Lyme disease. J. Infect. Dis. 189:1962-1964. [DOI] [PubMed] [Google Scholar]
- 138.Johnson, B. J., K. E. Robbins, R. E. Bailey, B. L. Cao, S. L. Sviat, R. B. Craven, L. W. Mayer, and D. T. Dennis. 1996. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J. Infect. Dis. 174:346-353. [DOI] [PubMed] [Google Scholar]
- 139.Johnston, Y. E., P. H. Duray, A. C. Steere, M. Kashgarian, J. Buza, S. E. Malawista, and P. W. Askenase. 1985. Lyme arthritis. Spirochetes found in synovial microangiopathic lesions. Am. J. Pathol. 118:26-34. [PMC free article] [PubMed] [Google Scholar]
- 140.Jurca, T., E. Ruzic-Sabljic, S. Lotric-Furlan, V. Maraspin, J. Cimperman, R. N. Picken, and F. Strle. 1998. Comparison of peripheral and central biopsy sites for the isolation of Borrelia burgdorferi sensu lato from erythema migrans skin lesions. Clin. Infect. Dis. 27:636-638. [DOI] [PubMed] [Google Scholar]
- 141.Kaiser, R. 2000. False-negative serology in patients with neuroborreliosis and the value of employing of different borrelial strains in serological assays. J. Med. Microbiol. 49:911-915. [DOI] [PubMed] [Google Scholar]
- 142.Kaiser, R., C. Rasiah, G. Gassmann, A. Vogt, and C. H. Lucking. 1993. Intrathecal antibody synthesis in Lyme neuroborreliosis: use of recombinant p41 and a 14-kDa flagellin fragment in ELISA. J. Med. Microbiol. 39:290-297. [DOI] [PubMed] [Google Scholar]
- 143.Kaiser, R., and S. Rauer. 1998. Analysis of the intrathecal immune response in neuroborreliosis to a sonicate antigen and three recombinant antigens of Borrelia burgdorferi sensu stricto. Eur. J. Clin. Microbiol. Infect. Dis. 17:159-166. [DOI] [PubMed] [Google Scholar]
- 144.Kaiser, R., and S. Rauer. 1999. Advantage of recombinant borrelial proteins for serodiagnosis of neuroborreliosis. J. Med. Microbiol. 48:5-10. [DOI] [PubMed] [Google Scholar]
- 145.Kalish, R. A., J. M. Leong, and A. C. Steere. 1993. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect. Immun. 61:2774-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karch, H., H. I. Huppertz, M. Bohme, H. Schmidt, D. Wiebecke, and A. Schwarzkopf. 1994. Demonstration of Borrelia burgdorferi DNA in urine samples from healthy humans whose sera contain B. burgdorferi-specific antibodies. J. Clin. Microbiol. 32:2312-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karlsson, M., K. Hovind-Hougen, B. Svenungsson, and G. Stiernstedt. 1990. Cultivation and characterization of spirochetes from cerebrospinal fluid of patients with Lyme borreliosis. J. Clin. Microbiol. 28:473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Karlsson, M., G. Stiernstedt, M. Granstrom, E. Asbrink, and B. Wretlind. 1990. Comparison of flagellum and sonicate antigens for serological diagnosis of Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 9:169-177. [DOI] [PubMed] [Google Scholar]
- 149.Kawabata, H., T. Masuzawa, and Y. Yanagihara. 1993. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol. Immunol. 37:843-848. [DOI] [PubMed] [Google Scholar]
- 150.Keller, T. L., J. J. Halperin, and M. Whitman. 1992. PCR detection of Borrelia burgdorferi DNA in cerebrospinal fluid of Lyme neuroborreliosis patients. Neurology 42:32-42. [DOI] [PubMed] [Google Scholar]
- 151.Kellogg, J. A., J. P. Manzella, and J. H. McConville. 1984. Clinical laboratory comparison of the 10-ml Isolator blood culture system with BACTEC radiometric blood culture media. J. Clin. Microbiol. 20:618-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kelly, R. 1971. Cultivation of Borrelia hermsii. Science 173:443-444. [DOI] [PubMed] [Google Scholar]
- 153.Klein, J., G. Stanek, R. Bittner, R. Horvat, C. Holzinger, and D. Glogar. 1991. Lyme borreliosis as a cause of myocarditis and heart muscle disease. Eur. Heart J. 12(Suppl. D):73-75. [DOI] [PubMed] [Google Scholar]
- 154.Klempner, M. S., L. T. Hu, J. Evans, C. H. Schmid, G. M. Johnson, R. P. Trevino, D. Norton, L. Levy, D. Wall, J. McCall, M. Kosinski, and A. Weinstein. 2001. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N. Engl. J. Med. 345:85-92. [DOI] [PubMed] [Google Scholar]
- 155.Klempner, M. S., C. H. Schmid, L. Hu, A. C. Steere, G. Johnson, B. McCloud, R. Noring, and A. Weinstein. 2001. Intralaboratory reliability of serologic and urine testing for Lyme disease. Am. J. Med. 110:217-219. [DOI] [PubMed] [Google Scholar]
- 156.Kornblatt, A. N., A. C. Steere, and D. G. Brownstein. 1984. Experimental Lyme disease in rabbits: spirochetes found in erythema migrans and blood. Infect. Immun. 46:220-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kurtti, T. J., U. G. Munderloh, G. G. Ahlstrand, and R. C. Johnson. 1988. Borrelia burgdorferi in tick cell culture: growth and cellular adherence. J. Med. Entomol. 25:256-261. [DOI] [PubMed] [Google Scholar]
- 158.Kurtti, T. J., U. G. Munderloh, R. C. Johnson, and G. G. Ahlstrand. 1987. Colony formation and morphology in Borrelia burgdorferi. J. Clin. Microbiol. 25:2054-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lagal, V., D. Postic, E. Ruzic-Sabljic, and G. Baranton. 2003. Genetic diversity among Borrelia strains determined by single-strand conformation polymorphism analysis of the ospC gene and its association with invasiveness. J. Clin. Microbiol. 41:5059-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lahdenne, P., J. Panelius, H. Saxen, T. Heikkila, H. Sillanpaa, M. Peltomaa, M. Arnez, H. I. Huppertz, and I. J. Seppala. 2003. Improved serodiagnosis of erythema migrans using novel recombinant borrelial BBK32 antigens. J. Med. Microbiol. 52:563-567. [DOI] [PubMed] [Google Scholar]
- 161.Lawrenz, M. B., J. M. Hardham, R. T. Owens, J. Nowakowski, A. C. Steere, G. P. Wormser, and S. J. Norris. 1999. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J. Clin. Microbiol. 37:3997-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lebech, A. M. 2002. Polymerase chain reaction in diagnosis of Borrelia burgdorferi infections and studies on taxonomic classification. APMIS Suppl. 105:1-40. [PubMed] [Google Scholar]
- 163.Lebech, A. M., O. Clemmensen, and K. Hansen. 1995. Comparison of in vitro culture, immunohistochemical staining, and PCR for detection of Borrelia burgdorferi in tissue from experimentally infected animals. J. Clin. Microbiol. 33:2328-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lebech, A. M., and K. Hansen. 1992. Detection of Borrelia burgdorferi DNA in urine samples and cerebrospinal fluid samples from patients with early and late Lyme neuroborreliosis by polymerase chain reaction. J. Clin. Microbiol. 30:1646-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lebech, A. M., K. Hansen, F. Brandrup, O. Clemmensen, and L. Halkier-Sorensen. 2000. Diagnostic value of PCR for detection of Borrelia burgdorferi DNA in clinical specimens from patients with erythema migrans and Lyme neuroborreliosis. Mol. Diagn. 5:139-150. [DOI] [PubMed] [Google Scholar]
- 166.Lebech, A. M., K. Hansen, B. J. Rutledge, C. P. Kolbert, P. N. Rys, and D. H. Persing. 1998. Diagnostic detection and direct genotyping of Borrelia burgdorferi regular by polymerase chain reaction in cerebrospinal fluid in Lyme neuroborreliosis. Mol. Diagn. 3:131-141. [DOI] [PubMed] [Google Scholar]
- 167.Ledue, T. B., M. F. Collins, and W. Y. Craig. 1996. New laboratory guidelines for serologic diagnosis of Lyme disease: evaluation of the two-test protocol. J. Clin. Microbiol. 34:2343-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Le Fleche, A., D. Postic, K. Girardet, O. Peter, and G. Baranton. 1997. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47:921-925. [DOI] [PubMed] [Google Scholar]
- 169.Liang, F. T., E. Aberer, M. Cinco, L. Gern, C. M. Hu, Y. N. Lobet, M. Ruscio, P. E. Voet, Jr., V. E. Weynants, and M. T. Philipp. 2000. Antigenic conservation of an immunodominant invariable region of the VlsE lipoprotein among European pathogenic genospecies of Borrelia burgdorferi SL. J. Infect. Dis. 182:1455-1462. [DOI] [PubMed] [Google Scholar]
- 170.Liang, F. T., A. L. Alvarez, Y. Gu, J. M. Nowling, R. Ramamoorthy, and M. T. Philipp. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol. 163:5566-5573. [PubMed] [Google Scholar]
- 171.Liang, F. T., A. C. Steere, A. R. Marques, B. J. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liebling, M. R., M. J. Nishio, A. Rodriguez, L. H. Sigal, T. Jin, and J. S. Louie. 1993. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum. 36:665-675. [DOI] [PubMed] [Google Scholar]
- 173.Lin, T., J. H. Oliver, Jr., L. Gao, T. M. Kollars, Jr., and K. L. Clark. 2001. Genetic heterogeneity of Borrelia burgdorferi sensu lato in the southern United States based on restriction fragment length polymorphism and sequence analysis. J. Clin. Microbiol. 39:2500-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin, T. M., C. M. Schubert, F. F. Shih, P. Ahmad, M. Lopez, and H. Horst. 1991. Use of flagellin-enriched antigens in a rapid, simple and specific quantitative enzyme immunoassay for Lyme disease antibodies in human serum samples. J. Immunoassay 12:325-346. [DOI] [PubMed] [Google Scholar]
- 175.Liveris, D., A. Gazumyan, and I. Schwartz. 1995. Molecular typing of Borrelia burgdorferi sensu lato by PCR-restriction fragment length polymorphism analysis. J. Clin. Microbiol. 33:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liveris, D., S. Varde, R. Iyer, S. Koenig, S. Bittker, D. Cooper, D. McKenna, J. Nowakowski, R. B. Nadelman, G. P. Wormser, and I. Schwartz. 1999. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J. Clin. Microbiol. 37:565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liveris, D., G. Wang, G. Girao, D. W. Byrne, J. Nowakowski, D. McKenna, R. Nadelman, G. P. Wormser, and I. Schwartz. 2002. Quantitative detection of Borrelia burgdorferi in 2-millimeter skin samples of erythema migrans lesions: correlation of results with clinical and laboratory findings. J. Clin. Microbiol. 40:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Logar, M., S. Lotric-Furlan, V. Maraspin, J. Cimperman, T. Jurca, E. Ruzic-Sabljic, and F. Strle. 1999. Has the presence or absence of Borrelia burgdorferi sensu lato as detected by skin culture any influence on the course of erythema migrans? Wien. Klin. Wochenschr. 111:945-950. [PubMed] [Google Scholar]
- 179.Luft, B. J., J. J. Dunn, R. J. Dattwyler, G. Gorgone, P. D. Gorevic, and W. H. Schubach. 1993. Cross-reactive antigenic domains of the flagellin protein of Borrelia burgdorferi. Res. Microbiol. 144:251-257. [DOI] [PubMed] [Google Scholar]
- 180.Luft, B. J., C. R. Steinman, H. C. Neimark, B. Muralidhar, T. Rush, M. F. X. K. M. Finkel, and R. J. Dattwyler. 1992. Invasion of the central nervous system by Borrelia burgdorferi in acute disseminated infection JAMA 267:1364-1367. (Erratum, 268:872.) [PubMed] [Google Scholar]
- 181.Ma, B., B. Christen, D. Leung, and C. Vigo-Pelfrey. 1992. Serodiagnosis of Lyme borreliosis by Western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J. Clin. Microbiol. 30:370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Magnarelli, L. A., E. Fikrig, R. Berland, J. F. Anderson, and R. A. Flavell. 1992. Comparison of whole-cell antibodies and an antigenic flagellar epitope of Borrelia burgdorferi in serologic tests for diagnosis of Lyme borreliosis. J. Clin. Microbiol. 30:3158-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Magnarelli, L. A., E. Fikrig, S. J. Padula, J. F. Anderson, and R. A. Flavell. 1996. Use of recombinant antigens of Borrelia burgdorferi in serologic tests for diagnosis of Lyme borreliosis. J. Clin. Microbiol. 34:237-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Magnarelli, L. A., J. W. Ijdo, S. J. Padula, R. A. Flavell, and E. Fikrig. 2000. Serologic diagnosis of lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 38:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Magnarelli, L. A., M. Lawrenz, S. J. Norris, and E. Fikrig. 2002. Comparative reactivity of human sera to recombinant VlsE and other Borrelia burgdorferi antigens in class-specific enzyme-linked immunosorbent assays for Lyme borreliosis. J. Med. Microbiol. 51:649-655. [DOI] [PubMed] [Google Scholar]
- 186.Magnarelli, L. A., J. M. Meegan, J. F. Anderson, and W. A. Chappell. 1984. Comparison of an indirect fluorescent-antibody test with an enzyme-linked immunosorbent assay for serological studies of Lyme disease. J. Clin. Microbiol. 20:181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Maiwald, M., C. Stockinger, D. Hassler, D. M. von Knebel, and H. G. Sonntag. 1995. Evaluation of the detection of Borrelia burgdorferi DNA in urine samples by polymerase chain reaction. Infection 23:173-179. [DOI] [PubMed] [Google Scholar]
- 188.Maraspin, V., J. Cimperman, S. Lotric-Furlan, E. Ruzic-Sabljic, T. Jurca, R. N. Picken, and F. Strle. 2002. Solitary borrelial lymphocytoma in adult patients. Wien. Klin. Wochenschrift. 114:515-523. [PubMed] [Google Scholar]
- 189.Maraspin, V., E. Ruzic-Sabljic, J. Cimperman, S. Lotric-Furlan, T. Jurca, R. N. Picken, and F. Strle. 2001. Isolation of Borrelia burgdorferi sensu lato from blood of patients with erythema migrans. Infection 29:65-70. [DOI] [PubMed] [Google Scholar]
- 190.Marcus, L. C., A. C. Steere, P. H. Duray, A. E. Anderson, and E. B. Mahoney. 1985. Fatal pancarditis in a patient with coexistent Lyme disease and babesiosis. Demonstration of spirochetes in the myocardium. Ann. Intern. Med. 103:374-376. [DOI] [PubMed] [Google Scholar]
- 191.Marques, A. R., F. Stock, and V. Gill. 2000. Evaluation of a new culture medium for Borrelia burgdorferi. J. Clin. Microbiol. 38:4239-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Massarotti, E. M., S. W. Luger, D. W. Rahn, R. P. Messner, J. B. Wong, R. C. Johnson, and A. C. Steere. 1992. Treatment of early Lyme disease. Am. J. Med. 92:396-403. [DOI] [PubMed] [Google Scholar]
- 193.Masuzawa, T., T. Kurita, H. Kawabata, and Y. Yanagihara. 1994. Relationship between infectivity and OspC expression in Lyme disease Borrelia. FEMS Microbiol. Lett. 123:319-324. [DOI] [PubMed] [Google Scholar]
- 194.Masuzawa, T., N. Takada, M. Kudeken, T. Fukui, Y. Yano, F. Ishiguro, Y. Kawamura, Y. Imai, and T. Ezaki. 2001. Borrelia sinica sp. nov., a Lyme disease-related Borrelia species isolated in China. Int. J. Syst. Evol. Microbiol. 51:1817-1824. [DOI] [PubMed] [Google Scholar]
- 195.Mathiesen, D. A., J. H. Oliver, Jr., C. P. Kolbert, E. D. Tullson, B. J. Johnson, G. L. Campbell, P. D. Mitchell, K. D. Reed, S. R. Telford III, J. F. Anderson, R. S. Lane, and D. H. Persing. 1997. Genetic heterogeneity of Borrelia burgdorferi in the United States. J. Infect. Dis. 175:98-107. [DOI] [PubMed] [Google Scholar]
- 196.Mathiesen, M. J., M. Christiansen, K. Hansen, A. Holm, E. Asbrink, and M. Theisen. 1998. Peptide-based OspC enzyme-linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 36:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mathiesen, M. J., K. Hansen, N. Axelsen, L. Halkier-Sorensen, and M. Theisen. 1996. Analysis of the human antibody response to outer surface protein C (OspC) of Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii. Med. Microbiol. Immunol. (Berlin) 185:121-129. [DOI] [PubMed] [Google Scholar]
- 198.Mathiesen, M. J., A. Holm, M. Christiansen, J. Blom, K. Hansen, S. Ostergaard, and M. Theisen. 1998. The dominant epitope of Borrelia garinii outer surface protein C recognized by sera from patients with neuroborreliosis has a surface-exposed conserved structural motif. Infect. Immun. 66:4073-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Melchers, W., J. Meis, P. Rosa, E. Claas, L. Nohlmans, R. Koopman, A. Horrevorts, and J. Galama. 1991. Amplification of Borrelia burgdorferi DNA in skin biopsies from patients with Lyme disease. J. Clin. Microbiol. 29:2401-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Melski, J. W., K. D. Reed, P. D. Mitchell, and G. D. Barth. 1993. Primary and secondary erythema migrans in central Wisconsin. Arch. Dermatol. 129:709-716. [PubMed] [Google Scholar]
- 201.Mermel, L. A., and D. G. Maki. 1993. Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann. Intern. Med. 119:270-272. [DOI] [PubMed] [Google Scholar]
- 202.Miller, J. C., K. von Lackum, K. Babb, J. D. McAlister, and B. Stevenson. 2003. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect. Immun. 71:6943-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mitchell, P. D., K. D. Reed, T. L. Aspeslet, M. F. Vandermause, and J. W. Melski. 1994. Comparison of four immunoserologic assays for detection of antibodies to Borrelia burgdorferi in patients with culture-positive erythema migrans. J. Clin. Microbiol. 32:1958-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mitchell, P. D., K. D. Reed, M. F. Vandermause, and J. W. Melski. 1993. Isolation of Borrelia burgdorferi from skin biopsy specimens of patients with erythema migrans. Am. J. Clin. Pathol. 99:104-107. [DOI] [PubMed] [Google Scholar]
- 205.Molloy, P. J., V. P. Berardi, D. H. Persing, and L. H. Sigal. 2000. Detection of multiple reactive protein species by immunoblotting after recombinant outer surface protein A Lyme disease vaccination. Clin. Infect. Dis. 31:42-47. [DOI] [PubMed] [Google Scholar]
- 206.Molloy, P. J., D. H. Persing, and V. P. Berardi. 2001. False-positive results of PCR testing for Lyme disease. Clin. Infect. Dis. 33:412-413. [DOI] [PubMed] [Google Scholar]
- 207.Mommert, S., R. Gutzmer, A. Kapp, and T. Werfel. 2001. Sensitive detection of Borrelia burgdorferi sensu lato DNA and differentiation of Borrelia species by LightCycler PCR. J. Clin. Microbiol. 39:2663-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Moter, S. E., H. Hofmann, R. Wallich, M. M. Simon, and M. D. Kramer. 1994. Detection of Borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by ospA-specific PCR. J. Clin. Microbiol. 32:2980-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Muellegger, R., N. Zoechling, E. M. Schluepen, H. P. Soyer, S. Hoedl, Kerl, and M. Volkenandt. 1996. Polymerase chain reaction control of antibiotic treatment in dermatoborreliosis. Infection 24:76-79. [DOI] [PubMed] [Google Scholar]
- 211.Muellegger, R. R., N. Zoechling, H. P. Soyer, S. Hoedl, R. Wienecke, M. Volkenandt, and H. Kerl. 1995. No detection of Borrelia burgdorferi-specific DNA in erythema migrans lesions after minocycline treatment. Arch. Dermatol. 131:678-682. [PubMed] [Google Scholar]
- 212.Muller-Felber, W., C. D. Reimers, J. de Koning, P. Fischer, A. Pilz, and D. E. Pongratz. 1993. Myositis in Lyme borreliosis: an immunohistochemical study of seven patients. J. Neurol. Sci. 118:207-212. [DOI] [PubMed] [Google Scholar]
- 213.Munderloh, U. G., Y. Liu, M. Wang, C. Chen, and T. J. Kurtti. 1994. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J. Parasitol. 80:533-543. [PubMed] [Google Scholar]
- 214.Nadelman, R. B., J. Nowakowski, G. Forseter, S. Bittker, D. Cooper, N. Goldberg, D. McKenna, and G. P. Wormser. 1993. Failure to isolate Borrelia burgdorferi after antimicrobial therapy in culture-documented Lyme borreliosis associated with erythema migrans: report of a prospective study. Am. J. Med. 94:583-588. [DOI] [PubMed] [Google Scholar]
- 215.Nadelman, R. B., J. Nowakowski, G. Forseter, N. S. Goldberg, S. Bittker, Cooper, M. Aguero-Rosenfeld, and G. P. Wormser. 1996. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am. J. Med. 100:502-508. [DOI] [PubMed] [Google Scholar]
- 216.Nadelman, R. B., C. S. Pavia, L. A. Magnarelli, and G. P. Wormser. 1990. Isolation of Borrelia burgdorferi from the blood of seven patients with Lyme disease. Am. J. Med. 88:21-26. [DOI] [PubMed] [Google Scholar]
- 217.Nadelman, R. B., I. Schwartz, and G. P. Wormser. 1994. Detecting Borrelia burgdorferi in blood from patients with Lyme disease. J. Infect. Dis. 169:1410-1411. [DOI] [PubMed] [Google Scholar]
- 218.Nadelman, R. B., and G. P. Wormser. 1995. Erythema migrans and early Lyme disease. Am. J. Med. 98:15S-23S. [DOI] [PubMed] [Google Scholar]
- 219.Nadelman, R. B., and G. P. Wormser. 1998. Lyme borreliosis. Lancet 352:557-565. [DOI] [PubMed] [Google Scholar]
- 220.Narasimhan, S., M. J. Camaino, F. T. Liang, F. Santiago, M. Laskowski, M. T. Philipp, A. R. Pachner, J. D. Radolf, and E. Fikrig. 2003. Borrelia burgdorferi transcriptome in the central nervous system of non-human primates. Proc. Natl. Acad. Sci. USA 100:15953-15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Neubert, U., H. E. Krampitz, and H. Engl. 1986. Microbiological findings in erythema (chronicum) migrans and related disorders. Zentbl. Bakteriol. Mikrobiol. Hyg. A 263:237-252. [DOI] [PubMed] [Google Scholar]
- 222.Nichol, G., D. T. Dennis, A. C. Steere, R. Lightfoot, G. Wells, B. Shea, and P. Tugwell. 1998. Test-treatment strategies for patients suspected of having Lyme disease: a cost-effectiveness analysis. Ann. Intern. Med. 128:37-48. [DOI] [PubMed] [Google Scholar]
- 223.Nocton, J. J., B. J. Bloom, B. J. Rutledge, D. H. Persing, E. L. Logigian, G. P. Schmid, and A. C. Steere. 1996. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in cerebrospinal fluid in Lyme neuroborreliosis. J. Infect. Dis. 174:623-627. [DOI] [PubMed] [Google Scholar]
- 224.Nocton, J. J., F. Dressler, B. J. Rutledge, P. N. Rys, D. H. Persing, and A. C. Steere. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 330:229-234. [DOI] [PubMed] [Google Scholar]
- 225.Nordstrand, A., A. G. Barbour, and S. Bergstrom. 2000. Borrelia pathogenesis research in the post-genomic and post-vaccine era. Curr. Opin. Microbiol. 3:86-92. [DOI] [PubMed] [Google Scholar]
- 226.Nowakowski, J., D. McKenna, R. B. Nadelman, D. Cooper, S. Bittker, D. Holmgren, C. Pavia, R. C. Johnson, and G. P. Wormser. 2000. Failure of treatment with cephalexin for Lyme disease. Arch. Fam. Med. 9:563-567. [DOI] [PubMed] [Google Scholar]
- 227.Nowakowski, J., I. Schwartz, D. Liveris, G. Wang, M. E. Aguero-Rosenfeld, G. Girao, D. McKenna, R. B. Nadelman, L. F. Cavaliere, and G. P. Wormser. 2001. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin. Infect. Dis. 33:2023-2027. [DOI] [PubMed] [Google Scholar]
- 228.Ntchobo, H., H. Rothermel, W. Chege, A. C. Steere, and J. Coburn. 2001. Recognition of multiple antibody epitopes throughout Borrelia burgdorferi p66, a candidate adhesin, in patients with early or late manifestations of Lyme disease. Infect. Immun. 69:1953-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Obonyo, M., U. G. Munderloh, V. Fingerle, B. Wilske, and T. J. Kurtti. 1999. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J. Clin. Microbiol. 37:2137-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ojaimi, C., B. E. Davidson, I. Saint Girons, and I. G. Old. 1994. Conservation of gene arrangement and an unusual organization of rRNA genes in the linear chromosomes of the Lyme disease spirochaetes Borrelia burgdorferi, B. garinii and B. afzelii. Microbiology 140:2931-2940. [DOI] [PubMed] [Google Scholar]
- 233.Oksi, J., M. Marjamaki, J. Nikoskelainen, and M. K. Viljanen. 1999. Borrelia burgdorferi detected by culture and PCR in clinical relapse of disseminated Lyme borreliosis. Ann. Med. 31:225-232. [DOI] [PubMed] [Google Scholar]
- 234.Oksi, J., H. Marttila, H. Soini, H. Aho, J. Uksila, and M. K. Viljanen. 2001. Early dissemination of Borrelia burgdorferi without generalized symptoms in patients with erythema migrans. APMIS 109:581-588. [DOI] [PubMed] [Google Scholar]
- 235.Oksi, J., J. Uksila, M. Marjamaki, J. Nikoskelainen, and M. K. Viljanen. 1995. Antibodies against whole sonicated Borrelia burgdorferi spirochetes, 41-kilodalton flagellin, and P39 protein in patients with PCR- or culture-proven late Lyme borreliosis. J. Clin. Microbiol. 33:2260-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ornstein, K., J. Berglund, S. Bergstrom, R. Norrby, and A. G. Barbour. 2002. Three major Lyme Borrelia genospecies (Borrelia burgdorferi sensu stricto, B. afzelii and B. garinii) identified by PCR in cerebrospinal fluid from patients with neuroborreliosis in Sweden. Scand. J. Infect. Dis. 34:341-346. [DOI] [PubMed] [Google Scholar]
- 237.Ornstein, K., J. Berglund, I. Nilsson, R. Norrby, and S. Bergstrom. 2001. Characterization of Lyme borreliosis isolates from patients with erythema migrans and neuroborreliosis in southern Sweden. J. Clin. Microbiol. 39:1294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Oschmann, P., W. Dorndorf, C. Hornig, C. Schafer, H. J. Wellensiek, and K. W. Pflughaupt. 1998. Stages and syndromes of neuroborreliosis. J. Neurol. 245:262-272. [DOI] [PubMed] [Google Scholar]
- 239.Pachner, A. R., and E. Delaney. 1993. The polymerase chain reaction in the diagnosis of Lyme neuroborreliosis. Ann. Neurol. 34:544-550. [DOI] [PubMed] [Google Scholar]
- 240.Pachner, A. R., A. C. Steere, L. H. Sigal, and C. J. Johnson. 1985. Antigen-specific proliferation of CSF lymphocytes in Lyme disease. Neurology 35:1642-1644. [DOI] [PubMed] [Google Scholar]
- 241.Padula, S. J., F. Dias, A. Sampieri, R. B. Craven, and R. W. Ryan. 1994. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 32:1733-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pahl, A., U. Kuhlbrandt, K. Brune, M. Rollinghoff, and A. Gessner. 1999. Quantitative detection of Borrelia burgdorferi by real-time PCR. J. Clin. Microbiol. 37:1958-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Palmer, N., C. Fraser, and S. Casjens. 2000. Distribution of twelve linear extrachromosomal DNAs in natural isolates of Lyme disease spirochetes. J. Bacteriol. 182:2476-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Panelius, J., P. Lahdenne, T. Heikkila, M. Peltomaa, J. Oksi, and I. Seppala. 2002. Recombinant OspC from Borrelia burgdorferi sensu stricto, B. afzelii and B. garinii in the serodiagnosis of Lyme borreliosis. J. Med. Microbiol. 51:731-739. [DOI] [PubMed] [Google Scholar]
- 245.Panelius, J., P. Lahdenne, H. Saxen, S. A. Carlsson, T. Heikkila, M. Peltomaa, A. Lauhio, and I. Seppala. 2003. Diagnosis of Lyme neuroborreliosis with antibodies to recombinant proteins DbpA, BBK32, and OspC, and VlsE IR6 peptide. J. Neurol. 250:1318-1327. [DOI] [PubMed] [Google Scholar]
- 246.Panelius, J., P. Lahdenne, H. Saxen, T. Heikkila, and I. Seppala. 2001. Recombinant flagellin A proteins from Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 39:4013-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Parola, P., and D. Raoult. 2001. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 32:897-928. [DOI] [PubMed] [Google Scholar]
- 248.Pavia, C. S., G. P. Wormser, S. Bittker, and D. Cooper. 2000. An indirect hemagglutination antibody test to detect antibodies to Borrelia burgdorferi in patients with Lyme disease. J. Microbiol. Methods 40:163-173. [DOI] [PubMed] [Google Scholar]
- 249.Pavia, C. S., G. P. Wormser, G. L. Mormser, and G. L. Norman. 1997. Activity of sera from patients with Lyme disease against Borrelia burgdorferi. Clin. Infect. Dis. 25:S25-30. [DOI] [PubMed] [Google Scholar]
- 250.Peltomaa, M., G. McHugh, and A. C. Steere. 2003. Persistence of the antibody response to the VlsE sixth invariant region (IR6) peptide of Borrelia burgdorferi after successful antibiotic treatment of Lyme disease. J. Infect. Dis. 187:1178-1186. [DOI] [PubMed] [Google Scholar]
- 251.Pennell, D. R., P. J. Wand, and R. F. Schell. 1987. Evaluation of a quantitative fluorescence immunoassay (FIAX) for detection of serum antibody to Borrelia burgdorferi. J. Clin. Microbiol. 25:2218-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Persing, D. H., B. J. Rutledge, P. N. Rys, D. S. Podzorski, P. D. Mitchell, K. D. X. L. B. Reed, E. Fikrig, and S. E. Malawista. 1994. Target imbalance: disparity of Borrelia burgdorferi genetic material in synovial fluid from Lyme arthritis patients. J. Infect. Dis. 169:668-672. [DOI] [PubMed] [Google Scholar]
- 253.Pfister, H. W., B. Wilske, and K. Weber. 1994. Lyme borreliosis: basic science and clinical aspects. Lancet 343:1013-1016. [DOI] [PubMed] [Google Scholar]
- 254.Philipp, M. T., M. K. Aydintug, R. P. Bohm, Jr., F. B. Cogswell, V. A. Dennis, H. N. Lanners, R. C. Lowrie, Jr., E. D. Roberts, M. D. Conway, M. Karacorlu, et al. 1993. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect. Immun. 61:3047-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Philipp, M. T., A. R. Marques, P. T. Fawcett, L. G. Dally, and D. S. Martin. 2003. C6 test as an indicator of therapy outcome for patients with localized or disseminated Lyme borreliosis. J. Clin. Microbiol. 41:4955-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Picken, M. M., R. N. Picken, D. Han, Y. Cheng, E. Ruzic-Sabljic, J. Cimperman, V. Maraspin, S. Lotric-Furlan, and F. Strle. 1997. A two year prospective study to compare culture and polymerase chain reaction amplification for the detection and diagnosis of Lyme borreliosis. Mol. Pathol. 50:186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Picken, R. N., Y. Cheng, F. Strle, and M. M. Picken. 1996. Patient isolates of Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities of strain 25015. J. Infect. Dis. 174:1112-1115. [DOI] [PubMed] [Google Scholar]
- 258.Picken, R. N., F. Strle, M. M. Picken, E. Ruzic-Sabljic, V. Maraspin, S. Lotric-Furlan, and J. Cimperman. 1998. Identification of three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B. garinii, and B. afzelii) among isolates from acrodermatitis chronica atrophicans lesions. J. Investig. Dermatol. 110:211-214. [DOI] [PubMed] [Google Scholar]
- 259.Piesman, J., G. O. Maupin, E. G. Campos, and C. M. Happ. 1991. Duration of adult female Ixodes dammini attachment and transmission of Borrelia burgdorferi, with description of a needle aspiration isolation method. J. Infect. Dis. 163:895-897. [DOI] [PubMed] [Google Scholar]
- 260.Piesman, J., B. S. Schneider, and N. S. Zeidner. 2001. Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors. J. Clin. Microbiol. 39:4145-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pietila, J., Q. He, J. Oksi, and M. K. Viljanen. 2000. Rapid differentiation of Borrelia garinii from Borrelia afzelii and Borrelia burgdorferi sensu stricto by Lightcycler fluorescence melting curve analysis of a PCR product of the recA gene. J. Clin. Microbiol. 38:2756-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pollack, R. J., S. R. Telford III, and A. Spielman. 1993. Standardization of medium for culturing Lyme disease spirochetes. J. Clin. Microbiol. 31:1251-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Postic, D., M. Assous, J. Belfaiza, and G. Baranton. 1996. Genetic diversity of Borrelia of Lyme borreliosis. Wien. Klin. Wochenschr. 108:748-751. [PubMed] [Google Scholar]
- 264.Postic, D., N. M. Ras, R. S. Lane, M. Hendson, and G. Baranton. 1998. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127). J. Clin. Microbiol. 36:3497-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Preac-Mursic, V., H. W. Pfister, H. Spiegel, R. Burk, B. Wilske, S. Reinhardt, and R. Bohmer. 1993. First isolation of Borrelia burgdorferi from an iris biopsy. J. Clin. Neuroophthalmol. 13:155-161. [PubMed] [Google Scholar]
- 266.Preac-Mursic, V., B. Wilske, and S. Reinhardt. 1991. Culture of Borrelia burgdorferi on six solid media. Eur. J. Clin. Microbiol. Infect. Dis. 10:1076-1079. [DOI] [PubMed] [Google Scholar]
- 267.Preac-Mursic, V., B. Wilske, and G. Schierz. 1986. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentbl. Bakteriol. Mikrobiol. Hyg. A 263:112-118. [DOI] [PubMed] [Google Scholar]
- 268.Preac-Mursic, V., B. Wilske, G. Schierz, H. W. Pfister, and K. M. Einhäupl. 1984. Repeated isolation of spirochetes from the cerebrospinal fluid of a patients with meningoradiculitis Bannwarth. Eur. J. Clin. Microbiol. 3:564-565. [DOI] [PubMed] [Google Scholar]
- 269.Priem, S., G. R. Burmester, T. Kamradt, K. Wolbart, M. G. Rittig, and A. Krause. 1998. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann. Rheum. Dis. 57:118-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Priem, S., M. G. Rittig, T. Kamradt, G. R. Burmester, and A. Krause. 1997. An optimized PCR leads to rapid and highly sensitive detection of Borrelia burgdorferi in patients with Lyme borreliosis. J. Clin. Microbiol. 35:685-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ramamoorthy, R., L. Povinelli, and M. T. Philipp. 1996. Molecular characterization, genomic arrangement, and expression of bmpD, a new member of the bmp class of genes encoding membrane proteins of Borrelia burgdorferi. Infect. Immun. 64:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ranki, A., E. Aavik, P. Peterson, K. Schauman, and P. Nurmilaakso. 1994. Successful amplification of DNA specific for Finnish Borrelia burgdorferi isolates in erythema chronicum migrans but not in circumscribed scleroderma lesions. J. Investig. Dermatol. 102:339-345. [DOI] [PubMed] [Google Scholar]
- 273.Rasiah, C., S. Rauer, G. S. Gassmann, and A. Vogt. 1994. Use of a hybrid protein consisting of the variable region of the Borrelia burgdorferi flagellin and part of the 83-kDa protein as antigen for serodiagnosis of Lyme disease. J. Clin. Microbiol. 32:1011-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rasiah, C., E. Schiltz, J. Reichert, and A. Vogt. 1992. Purification and characterization of a tryptic peptide of Borrelia burgdorferi flagellin, which reduces cross-reactivity in immunoblots and ELISA. J. Gen. Microbiol. 138:147-154. [DOI] [PubMed] [Google Scholar]
- 275.Rauer, S., N. Spohn, C. Rasiah, U. Neubert, and A. Vogt. 1998. Enzyme-linked immunosorbent assay using recombinant OspC and the internal 14-kDa flagellin fragment for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 36:857-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rauter, C., R. Oehme, I. Diterich, M. Engele, and T. Hartung. 2002. Distribution of clinically relevant borrelia genospecies in ticks assessed by a novel, single-run, real-time PCR. J. Clin. Microbiol. 40:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Reed, K. D. 2002. Laboratory testing for Lyme disease: possibilities and practicalities. J. Clin. Microbiol. 40:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rijpkema, S. G., D. J. Tazelaar, M. Molkenboer, G. T. Noordhoek, G. Plantinga, L. M. Schouls, and J. F. Schellekens. 1997. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermitis chronica atrophicans. Clin. Microbiol. Infect. 3:109-116. [DOI] [PubMed] [Google Scholar]
- 280.Robertson, J., E. Guy, N. Andrews, B. Wilske, P. Anda, M. Granstrom, U. Hauser, Y. Moosmann, V. Sambri, J. Schellekens, G. Stanek, and J. Gray. 2000. A European multicenter study of immunoblotting in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 38:2097-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Roessler, D., U. Hauser, and B. Wilske. 1997. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J. Clin. Microbiol. 35:2752-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rosa, P. A., and T. G. Schwan. 1989. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J. Infect. Dis. 160:1018-1029. [DOI] [PubMed] [Google Scholar]
- 283.Rosa, P. A., K. Tilly, and P. E. Stewart. 2005. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat. Rev. Microbiol. 3:129-143. [DOI] [PubMed] [Google Scholar]
- 284.Russell, H., J. S. Sampson, G. P. Schmid, H. W. Wilkinson, and B. Plikaytis. 1984. Enzyme-linked immunosorbent assay and indirect immunofluorescence assay for Lyme disease. J. Infect. Dis. 149:465-470. [DOI] [PubMed] [Google Scholar]
- 285.Ruzic-Sabljic, E., S. Lotric-Furlan, V. Maraspin, J. Cimperman, D. Pleterski-Rigler, and F. Strle. 2001. Analysis of Borrelia burgdorferi sensu lato isolated from cerebrospinal fluid. APMIS 109:707-713. [DOI] [PubMed] [Google Scholar]
- 286.Sadziene, A., M. Jonsson, S. Bergstrom, R. K. Bright, R. C. Kennedy, and A. G. Barbour. 1994. A bactericidal antibody to Borrelia burgdorferi is directed against a variable region of the OspB protein. Infect. Immun. 62:2037-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sadziene, A., P. A. Thompson, and A. G. Barbour. 1993. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J. Infect. Dis. 167:165-172. [DOI] [PubMed] [Google Scholar]
- 288.Saint Girons, I., L. Gern, J. S. Gray, E. C. Guy, E. Korenberg, P. A. Nuttall, S. G. Rijpkema, A. Schonberg, G. Stanek, and D. Postic. 1998. Identification of Borrelia burgdorferi sensu lato species in Europe. Zentbl. Bakteriol. 287:190-195. [DOI] [PubMed] [Google Scholar]
- 289.Schmidli, J., T. Hunziker, P. Moesli, and U. B. Schaad. 1988. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J. Infect. Dis. 158:905-906. (Letter.) [DOI] [PubMed] [Google Scholar]
- 290.Schmidt, B., R. R. Muellegger, C. Stockenhuber, H. P. Soyer, S. Hoedl, and A. X. K. H. Luger. 1996. Detection of Borrelia burgdorferi-specific DNA in urine specimens from patients with erythema migrans before and after antibiotic therapy. J. Clin. Microbiol. 34:1359-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Schmidt, B. L. 1997. PCR in laboratory diagnosis of human Borrelia burgdorferi infections. Clin. Microbiol. Rev. 10:185-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Schmidt, B. L., E. Aberer, C. Stockenhuber, H. Klade, F. Breier, and A. Luger. 1995. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in the urine and breast milk of patients with Lyme borreliosis. Diagn. Microbiol. Infect. Dis. 21:121-128. [DOI] [PubMed] [Google Scholar]
- 293.Schnarr, S., N. Putschky, M. C. Jendro, H. Zeidler, M. Hammer, J. G. Kuipers, and J. Wollenhaupt. 2001. Chlamydia and Borrelia DNA in synovial fluid of patients with early undifferentiated oligoarthritis: results of a prospective study. Arthritis Rheum. 44:2679-2685. [DOI] [PubMed] [Google Scholar]
- 294.Schulte-Spechtel, U., G. Lehnert, G. Liegl, V. Fingerle, C. Heimerl, B. J. Johnson, and B. Wilske. 2003. Significant improvement of the recombinant Borrelia-specific immunoglobulin G immunoblot test by addition of VlsE and a DbpA homologue derived from Borrelia garinii for diagnosis of early neuroborreliosis. J. Clin. Microbiol. 41:1299-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Schutzer, S. E., P. K. Coyle, A. L. Belman, M. G. Golightly, and J. Drulle. 1990. Sequestration of antibody to Borrelia burgdorferi in immune complexes in seronegative Lyme disease. Lancet 335:312-315. [DOI] [PubMed] [Google Scholar]
- 296.Schwaiger, M., O. Peter, and P. Cassinotti. 2001. Routine diagnosis of Borrelia burgdorferi (sensu lato) infections using a real-time PCR assay. Clin. Microbiol. Infect. 7:461-469. [DOI] [PubMed] [Google Scholar]
- 297.Schwan, T. G. 1996. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect. Agents Dis. 5:167-181. [PubMed] [Google Scholar]
- 298.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Schwan, T. G., and J. Piesman. 2002. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 8:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Schwartz, I., S. Bittker, S. L. Bowen, D. Cooper, C. Pavia, and G. P. Wormser. 1993. Polymerase chain reaction amplification of culture supernatants for rapid detection of Borrelia burgdorferi. Eur. J. Clin. Microbiol. Infect. Dis. 12:879-882. [DOI] [PubMed] [Google Scholar]
- 302.Schwartz, I., S. Varde, R. B. Nadelman, G. P. Wormser, and D. Fish. 1997. Inhibition of efficient polymerase chain reaction amplification of Borrelia burgdorferi DNA in blood-fed ticks. Am. J. Trop. Med. Hyg. 56:339-342. [DOI] [PubMed] [Google Scholar]
- 303.Schwartz, I., G. P. Wormser, J. J. Schwartz, D. Cooper, P. Weissensee, A. Gazumyan, E. Zimmermann, N. S. Goldberg, S. Bittker, G. L. Campbell, et al. 1992. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J. Clin. Microbiol. 30:3082-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Seinost, G., D. E. Dykhuizen, R. J. Dattwyler, W. T. Golde, J. J. Dunn, I. N. Wang, G. P. Wormser, M. E. Schriefer, and B. J. Luft. 1999. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 67:3518-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sigal, L. H., A. C. Steere, D. H. Freeman, and J. M. Dwyer. 1986. Proliferative responses of mononuclear cells in Lyme disease. Reactivity to Borrelia burgdorferi antigens is greater in joint fluid than in blood. Arthritis Rheum. 29:761-769. [DOI] [PubMed] [Google Scholar]
- 306.Simpson, W. J., M. E. Schrumpf, and T. G. Schwan. 1990. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J. Clin. Microbiol. 28:1329-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Singh, S. K., and H. J. Girschick. 2004. Molecular survival strategies of the Lyme disease spirochete Borrelia burgdorferi. Lancet Infect. Dis. 4:575-583. [DOI] [PubMed] [Google Scholar]
- 308.Sivak, S. L., M. E. Aguero-Rosenfeld, J. Nowakowski, R. B. Nadelman, and G. P. Wormser. 1996. Accuracy of IgM immunoblotting to confirm the clinical diagnosis of early Lyme disease. Arch. Intern. Med. 156:2105-2109. [PubMed] [Google Scholar]
- 309.Smith, R. P., R. T. Schoen, D. W. Rahn, V. K. Sikand, J. Nowakowski, D. L. Parenti, M. S. Holman, D. H. Persing, and A. C. Steere. 2002. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann. Intern. Med. 136:421-428. [DOI] [PubMed] [Google Scholar]
- 310.Snydman, D. R., D. P. Schenkein, V. P. Berardi, C. C. Lastavica, and K. M. Pariser. 1986. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann. Intern. Med. 104:798-800. [DOI] [PubMed] [Google Scholar]
- 311.Speck, S., K. Failing, B. Reiner, and M. M. Wittenbrink. 2002. Evaluation of different media and a BGM cell culture assay for isolation of Borrelia burgdorferi sensu lato from ticks and dogs. Vet. Microbiol. 89:291-302. [DOI] [PubMed] [Google Scholar]
- 312.Stanek, G., J. Klein, R. Bittner, and D. Glogar. 1990. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N. Engl. J. Med. 322:249-252. [DOI] [PubMed] [Google Scholar]
- 313.Stanek, G., S. O'Connell, M. Cimmino, E. Aberer, W. Kristoferitsch, M. Granstrom, E. Guy, and J. Gray. 1996. European union concerted action on risk assessment in Lyme borreliosis: clinical case definitions for Lyme borreliosis. Wien. Klin. Wochenschr. 108:741-747. [PubMed] [Google Scholar]
- 314.Stanek, G., and F. Strle. 2003. Lyme borreliosis. Lancet 362:1639-1647. [DOI] [PubMed] [Google Scholar]
- 315.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 263:201-205. [DOI] [PubMed] [Google Scholar]
- 316.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 317.Steere, A. C., N. H. Bartenhagen, and J. E. Craft. 1983. The early clinical manifestations of Lyme disease. Ann. Intern. Med. 99:76-82. [DOI] [PubMed] [Google Scholar]
- 318.Steere, A. C., W. P. Batsford, M. Weinberg, J. Alexander, H. J. Berger, and S. X. M. SE. Wolfson. 1980. Lyme carditis: cardiac abnormalities of Lyme disease. Ann. Intern. Med. 93:8-16. [DOI] [PubMed] [Google Scholar]
- 319.Steere, A. C., T. F. Broderick, and S. E. Malawista. 1978. Erythema chronicum migrans and Lyme arthritis: epidemiologic evidence for a tick vector. Am. J. Epidemiol. 108:312-321. [DOI] [PubMed] [Google Scholar]
- 320.Steere, A. C., B. Falk, E. E. Drouin, L. A. Baxter-Lowe, J. Hammer, and G. T. Nepom. 2003. Binding of outer surface protein A and human lymphocyte function-associated antigen 1 peptides to HLA-DR molecules associated with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 48:534-540. [DOI] [PubMed] [Google Scholar]
- 321.Steere, A. C., and L. Glickstein. 2004. Elucidation of Lyme arthritis. Nat. Rev. Immunol. 4:143-152. [DOI] [PubMed] [Google Scholar]
- 322.Steere, A. C., R. L. Grodzicki, J. E. Craft, M. Shrestha, A. N. Kornblatt, and S. E. Malawista. 1984. Recovery of Lyme disease spirochetes from patients. Yale J. Biol. Med. 57:557-560. [PMC free article] [PubMed] [Google Scholar]
- 323.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 324.Steere, A. C., S. E. Malawista, J. A. Hardin, S. Ruddy, W. Askenase, and W. A. Andiman. 1977. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann. Intern. Med. 86:685-698. [DOI] [PubMed] [Google Scholar]
- 325.Steere, A. C., S. E. Malawista, D. R. Snydman, R. E. Shope, W. A. Andiman, M. R. Ross, and F. M. Steele. 1977. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 20:7-17. [DOI] [PubMed] [Google Scholar]
- 326.Steere, A. C., R. T. Schoen, and E. Taylor. 1987. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 107:725-731. [DOI] [PubMed] [Google Scholar]
- 327.Steere, A. C., V. K. Sikand, F. Meurice, D. L. Parenti, E. Fikrig, R. T. Schoen, J. Nowakowski, C. H. Schmid, S. Laukamp, C. Buscarino, and D. S. Krause. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N. Engl. J. Med. 339:209-215. [DOI] [PubMed] [Google Scholar]
- 328.Stoenner, H. G. 1974. Biology of Borrelia hermsii in Kelly medium. Appl. Microbiol. 28:540-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Stoenner, H. G., T. Dodd, and C. Larsen. 1982. Antigenic variation of Borrelia hermsii. J. Exp. Med. 156:1297-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Straubinger, R. K. 2000. PCR-based quantification of Borrelia burgdorferi organisms in canine tissues over a 500-day postinfection period. J. Clin. Microbiol. 38:2191-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Straubinger, R. K., A. F. Straubinger, B. A. Summers, and R. H. Jacobson. 2000. Status of Borrelia burgdorferi infection after antibiotic treatment and the effects of corticosteroids: an experimental study. J. Infect. Dis. 181:1069-1081. [DOI] [PubMed] [Google Scholar]
- 332.Strle, F., R. B. Nadelman, J. Cimperman, J. Nowakowski, R. N. Picken, I. Schwartz, V. Maraspin, M. E. Aguero-Rosenfeld, S. Varde, S. Lotric-Furlan, and G. P. Wormser. 1999. Comparison of culture-confirmed erythema migrans caused by Borrelia burgdorferi sensu stricto in New York State and by Borrelia afzelii in Slovenia. Ann. Intern. Med. 130:32-36. [DOI] [PubMed] [Google Scholar]
- 333.Strle, F., J. A. Nelson, E. Ruzic-Sabljic, J. Cimperman, V. Maraspin, S. Lotric-Furlan, Y. Cheng, M. M. Picken, G. M. Trenholme, and R. N. Picken. 1996. European Lyme borreliosis: 231 culture-confirmed cases involving patients with erythema migrans. Clin. Infect. Dis. 23:61-65. [DOI] [PubMed] [Google Scholar]
- 334.Strle, F., R. N. Picken, Y. Cheng, J. Cimperman, V. Maraspin, S. Lotric-Furlan, E. Ruzic-Sabljic, and M. M. Picken. 1997. Clinical findings for patients with Lyme borreliosis causing by Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities to strain 25015. Clin. Infect. Dis. 25:273-280. [DOI] [PubMed] [Google Scholar]
- 335.Szczepanski, A., and J. L. Benach. 1991. Lyme borreliosis: host responses to Borrelia burgdorferi. Microbiol. Rev. 55:21-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Theisen, M., B. Frederiksen, A. M. Lebech, J. Vuust, and K. Hansen. 1993. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J. Clin. Microbiol. 31:2570-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Trevejo, R. T., P. J. Krause, V. K. Sikand, M. E. Schriefer, R. Ryan, T. Lepore, W. Porter, and D. T. Dennis. 1999. Evaluation of two-test serodiagnostic method for early Lyme disease in clinical practice. J. Infect. Dis. 179:931-938. [DOI] [PubMed] [Google Scholar]
- 338.Trollmo, C., A. L. Meyer, A. C. Steere, D. A. Hafler, and B. T. Huber. 2001. Molecular mimicry in Lyme arthritis demonstrated at the single cell level: LFA-1 alpha L is a partial agonist for outer surface protein A-reactive T cells. J. Immunol. 166:5286-5291. [DOI] [PubMed] [Google Scholar]
- 339.Tugwell, P., D. T. Dennis, A. Weinstein, G. Wells, B. Shea, G. Nichol, R. Hayward, R. Lightfoot, P. Baker, and A. C. Steere. 1997. Laboratory evaluation in the diagnosis of Lyme disease. Ann. Intern. Med. 127:1109-1123. [DOI] [PubMed] [Google Scholar]
- 340.Tumani, H., G. Nolker, and H. Reiber. 1995. Relevance of cerebrospinal fluid variables for early diagnosis of neuroborreliosis. Neurology 45:1663-1670. [DOI] [PubMed] [Google Scholar]
- 341.Tylewska-Wierzbanowska, S., and T. Chmielewski. 1997. The isolation of Borrelia burgdorferi spirochetes from clinical material in cell line cultures. Zentbl. Bakteriol. 286:363-370. [DOI] [PubMed] [Google Scholar]
- 342.van Dam, A. P., H. Kuiper, K. Vos, A. Widjojokusumo, B. M. de Jongh, L. Spanjaard, A. C. Ramselaar, M. D. Kramer, and J. Dankert. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708-717. [DOI] [PubMed] [Google Scholar]
- 343.van der Linde, M. R. 1991. Lyme carditis: clinical characteristics of 105 cases. Scand. J. Infect. Dis. Suppl. 77:81-84. [PubMed] [Google Scholar]
- 344.von Stedingk, L. V., I. Olsson, H. S. Hanson, E. Asbrink, and A. Hovmark. 1995. Polymerase chain reaction for detection of Borrelia burgdorferi DNA in skin lesions of early and late Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 14:1-5. [DOI] [PubMed] [Google Scholar]
- 345.Wallach, F. R., A. L. Forni, J. Hariprashad, M. Y. Stoeckle, C. R. Steinberg, L. Fisher, S. E. Malawista, and H. W. Murray. 1993. Circulating Borrelia burgdorferi in patients with acute Lyme disease: results of blood cultures and serum DNA analysis. J. Infect. Dis. 168:1541-1543. [DOI] [PubMed] [Google Scholar]
- 346.Wang, G. 2002. Direct detection methods for the Lyme Borrelia, including the use of quantitative assays. Vector Borne Zoonotic Dis. 2:227-235. [DOI] [PubMed] [Google Scholar]
- 347.Wang, G., D. Liveris, B. Brei, H. Wu, R. C. Falco, D. Fish, and I. Schwartz. 2003. Real-time PCR for simultaneous detection and quantification of Borrelia burgdorferi in field-collected Ixodes scapularis ticks from the northeastern United States. Appl. Environ. Microbiol. 69:4561-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wang, G., C. Ojaimi, R. Iyer, V. Saksenberg, S. A. McClain, G. P. Wormser, and I. Schwartz. 2001. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect. Immun. 69:4303-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wang, G., C. Ojaimi, H. Wu, V. Saksenberg, R. Iyer, D. Liveris, S. A. McClain, G. P. Wormser, and I. Schwartz. 2002. Disease severity in a murine model of lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Wang, G., A. P. van Dam, A. Le Fleche, D. Postic, O. Peter, G. Baranton, R. de Boer, L. Spanjaard, and J. Dankert. 1997. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19). Int. J. Syst. Bacteriol. 47:926-932. [DOI] [PubMed] [Google Scholar]
- 351.Wang, G., A. P. van Dam, I. Schwartz, and J. Dankert. 1999. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev. 12:633-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wang, G., A. P. van Dam, L. Spanjaard, and J. Dankert. 1998. Molecular typing of Borrelia burgdorferi sensu lato by randomly amplified polymorphic DNA fingerprinting analysis. J. Clin. Microbiol. 36:768-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Wang, G., G. P. Wormser, and I. Schwartz. 2001. Borrelia burgdorferi, p. 2059-2092. In M. Sussman (ed.), Molecular medical microbiology. Academic Press, London, United Kingdom.
- 354.Washington, J. A., and D. M. Ilstrup. 1986. Blood cultures: issues and controversies. Rev. Infect. Dis. 8:792-802. [DOI] [PubMed] [Google Scholar]
- 355.Weber, K., and H. W. Pfister. 1993. History of Lyme borreliosis in Europe, p. 1-20. In K. Weber and W. Burgdorfer (ed.), Aspects of Lyme borreliosis. Springer-Verlag, Berlin, Germany.
- 356.Weinstein, M. P. 1996. Current blood culture methods and systems: clinical concepts, technology, and interpretation of results. Clin. Infect. Dis. 23:40-46. [DOI] [PubMed] [Google Scholar]
- 357.Weis, J. J. 2002. Host-pathogen interactions and the pathogenesis of murine Lyme disease. Curr. Opin. Rheumatol. 14:399-403. [DOI] [PubMed] [Google Scholar]
- 358.Wienecke, R., U. Neubert, and M. Volkenandt. 1993. Molecular detection of Borrelia burgdorferi in formalin-fixed, paraffin-embedded lesions of Lyme disease. J. Cutan. Pathol. 20:385-388. [DOI] [PubMed] [Google Scholar]
- 359.Wienecke, R., E. M. Schlupen, N. Zochling, U. Neubert, M. Meurer, and M. Volkenandt. 1995. No evidence for Borrelia burgdorferi-specific DNA in lesions of localized scleroderma. J. Investig. Dermatol. 104:23-26. [DOI] [PubMed] [Google Scholar]
- 360.Wilske, B. 2003. Diagnosis of Lyme borreliosis in Europe. Vector Borne Zoonotic Dis. 3:215-227. [DOI] [PubMed] [Google Scholar]
- 361.Wilske, B., S. Jauris-Heipke, R. Lobentanzer, I. Pradel, V. Preac-Mursic, D. Rössler, E. Soutschek, and R. C. Johnson. 1995. Phenotypic analysis of outer surface protein C (OspC) of Borrelia burgdorferi sensu lato by monoclonal antibodies: relationship to genospecies and OspA serotype. J. Clin. Microbiol. 33:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wilske, B., and V. Preac-Mursic. 1993. Microbiological diagnosis of Lyme borreliosis, p. 267-300. In K. Weber and W. Burgdorfer (ed.), Aspects of Lyme borreliosis. Springer-Verlag, Berlin, Germany.
- 363.Wilske, B., V. Preac-Mursic, G. Schierz, and K. V. Busch. 1986. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentbl. Bakteriol. A 263:92-102. [DOI] [PubMed] [Google Scholar]
- 364.Wilske, B., G. Schierz, V. Preac-Mursic, K. Von Busch, R. Kuhbeck, H. W. Pfister, and K. M. Einhäupl. 1986. Intrathecal production of specific antibodies against Borrelia burgdorferi in patients with lymphocytic meningoradiculitits. J. Infect. Dis. 153:304-314. [DOI] [PubMed] [Google Scholar]
- 365.Wilske, B., and M. E. Schriefer. 2003. Borrelia, p. 937-954. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Mannual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 366.Wormser, G. P., M. E. Aguero-Rosenfeld, and R. B. Nadelman. 1999. Lyme disease serology: problems and opportunities. JAMA 282:79-80. [DOI] [PubMed] [Google Scholar]
- 367.Wormser, G. P., S. Bittker, D. Cooper, J. Nowakowski, R. B. Nadelman, and C. Pavia. 2000. Comparison of the yields of blood cultures using serum or plasma from patients with early Lyme disease. J. Clin. Microbiol. 38:1648-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Wormser, G. P., S. Bittker, D. Cooper, J. Nowakowski, R. B. Nadelman, and C. Pavia. 2001. Yield of large-volume blood cultures in patients with early Lyme disease. J. Infect. Dis. 184:1070-1072. [DOI] [PubMed] [Google Scholar]
- 369.Wormser, G. P., G. Forseter, D. Cooper, J. Nowakowski, R. B. Nadelman, H. Horowitz, I. Schwartz, S. L. Bowen, G. L. Campbell, and N. S. Goldberg. 1992. Use of a novel technique of cutaneous lavage for diagnosis of Lyme disease associated with erythema migrans. JAMA 268:1311-1313. [PubMed] [Google Scholar]
- 369a.Wormser, G. P., C. Carbonaro, S. Miller, J. Nowakowski, R. B. Nadelman, S. Sivak, and M. E. Aguero-Rosenfeld. 2000. A limitation of 2-stage serological testing for Lyme disease: enzyme immunoassay and immunoblot assay are not independent tests. Clin. Infect. Dis. 30:545-548. [DOI] [PubMed] [Google Scholar]
- 370.Wormser, G. P., D. Liveris, J. Nowakowski, R. B. Nadelman, L. F. Cavaliere, D. McKenna, D. Holmgren, and I. Schwartz. 1999. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J. Infect. Dis. 180:720-725. [DOI] [PubMed] [Google Scholar]
- 371.Wormser, G. P., D. McKenna, J. Carlin, F. L. Cavaliere, D. Holmgren, D. W. Byrne, and J. Nowakowski. 2005. Hematogenous dissemination in early Lyme disease. Ann. Intern. Med. 142:751-755. [DOI] [PubMed] [Google Scholar]
- 372.Wormser, G. P., R. B. Nadelman, R. J. Dattwyler, D. T. Dennis, E. D. Shapiro, A. C. Steere, T. J. Rush, D. W. Rahn, P. K. Coyle, D. H. Persing, D. Fish, and B. J. Luft. 2000. Practice guidelines for the treatment of Lyme disease. Infect. Dis. Soc. Am. Clin. Infect. Dis. 31(Suppl. 1):1-14. [DOI] [PubMed] [Google Scholar]
- 373.Wormser, G. P., R. B. Nadelman, J. Nowakowski, and I. Schwartz. 2001. Asymptomatic Borrelia burgdorferi infection. Med. Hypotheses 57:435-438. [DOI] [PubMed] [Google Scholar]
- 374.Wormser, G. P., J. Nowakowski, R. B. Nadelman, S. Bittker, D. Cooper, and C. Pavia. 1998. Improving the yield of blood cultures for patients with early Lyme disease. J. Clin. Microbiol. 36:296-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Wormser, G. P., R. Ramanathan, J. Nowakowski, D. McKenna, D. Holmgren, P. Visintainer, R. Dornbush, B. Singh, and R. B. Nadelman. 2003. Duration of antibiotic therapy for early Lyme disease. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 138:697-704. [DOI] [PubMed] [Google Scholar]
- 376.Yang, X., T. G. Popova, K. E. Hagman, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 1999. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect. Immun. 67:6008-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zbinden, R., D. Goldenberger, G. M. Lucchini, and M. Altwegg. 1994. Comparison of two methods for detecting intrathecal synthesis of Borrelia burgdorferi-specific antibodies and PCR for diagnosis of Lyme neuroborreliosis. J. Clin. Microbiol. 32:1795-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]
- 379.Zhong, W., P. Oschmann, and H. J. Wellensiek. 1997. Detection and preliminary characterization of circulating immune complexes in patients with Lyme disease. Med. Microbiol. Immunol. (Berlin) 186:153-158. [DOI] [PubMed] [Google Scholar]
- 380.Zore, A., E. Ruzic-Sabljic, V. Maraspin, J. Cimperman, S. Lotric-Furlan, A. Pikelj, T. Jurca, M. Logar, and F. Strle. 2002. Sensitivity of culture and polymerase chain reaction for the etiologic diagnosis of erythema migrans. Wien. Klin. Wochenschrift. 114:606-609. [PubMed] [Google Scholar]