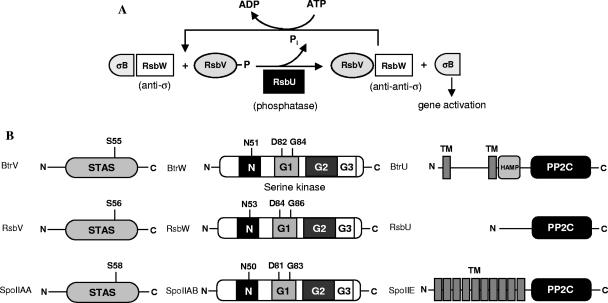
FIG. 1.
Partner switchers. (A) The B. subtilis partner-switching cycle regulates σB activity in the general stress response pathway (see the text). (B) Predicted protein structures of Bordetella BtrV, BtrW, and BtrU compared with B. subtilis orthologs. The conserved residues of BtrV and BtrW that were mutated and analyzed in this paper are shown. BtrV contains an anti-σ factor antagonist domain (STAS). BtrW contains an HPK-like serine kinase domain that forms a Bergerat fold with four motifs, called N, G1, G2, and G3 boxes, participating in binding to nucleotide ligands. BtrU contains two putative transmembrane domains separated by a stretch of mainly hydrophilic residues, a HAMP domain, and a PP2C-like serine phosphatase domain.