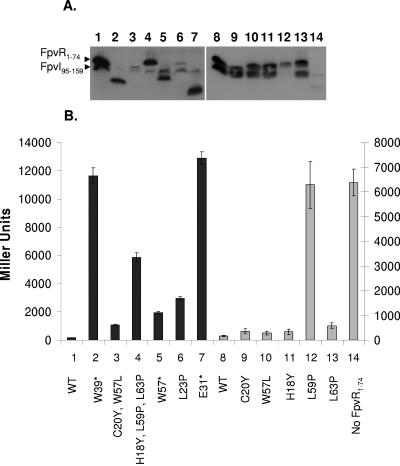
FIG. 5.
Impact of mutations in FpvR1-74 on its interaction with FpvI95-159. Western immunoblot of whole-cell extracts of E. coli SU202 carrying pAR014 (pDP804::fpvI95-159) and pMS604 (lane 14) or its derivatives expressing wild-type (lanes 1 and 8) or mutated (lanes 2 to 7 and 9 to 13) FpvR1-74 developed with antibodies to LexA. Specific mutations are indicated and numbered in panel B with the numbers used to match the mutation and β-galactosidase data to the corresponding immunoblot lane in panel A. (B) β-Galactosidase activity of E. coli SU202 carrying pMS604 or derivatives thereof expressing the indicated mutant FpvR1-74 proteins and pAR014 expressing FpvI95-159. Asterisks indicate the presence of a nonsense mutation in the corresponding fpvR codon, leading to truncation of the protein. WT, wild-type FpvR1-74. The scale to the left applies to the darkly shaded bars (1 to 7), while the scale to the right applies to the lightly shaded bars (8 to 14). Variability in protein yields (A) and β-galactosidase activity (B) for FpvI and FpvR derivatives shown in lanes 1 to 6 versus 7 to 14 reflects the fact that these data were obtained from experiments carried out independently on different days. Immunoblotting and β-galactosidase assays were performed on samples prepared from the same culture in all instances, and the data shown are representative of three replicates.