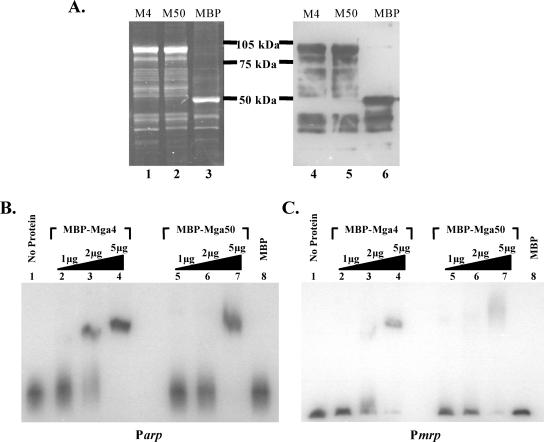
FIG. 2.
Electrophoretic mobility shift analysis of M4 and M50 MBP-Mga binding to class II Mga-regulated promoters. (A) Purification of M4 and M50 MBP-Mga fusion proteins from E. coli lysates using an amylose affinity resin. Purified protein was assessed with SDS-polyacrylamide gels stained with Sypro Ruby (left) and a Western blot probed with anti-MBP (right). Purified M4 MBP-Mga (lanes 1 and 4), M50 MBP-Mga (lanes 2 and 5), and MBP control (lanes 3 and 6) are shown. (B and C) EMSAs were performed on two M4 Mga-regulated promoters, Parp (B) and Pmrp (C). Identical amounts of each radiolabeled promoter probe were incubated for 15 min at 16°C with an increasing amount (1, 2, and 5 μg) of either the purified M4 MBP-Mga (lanes 2 to 4) or M50 MBP-Mga (lanes 5 to 7) fusion protein before being separated on a 5% polyacrylamide gel. Binding was also assessed in the presence of no protein (lanes 1) or 5 μg MBP alone (lanes 8) for each.