Abstract
Glutathione protects cells and organisms from oxygen species and peroxides and is indispensable for aerobically living organisms. Moreover, it acts against xenobiotics and drugs by the formation and excretion of glutathione S conjugates. In this study, we show that the yliA, -B, -C, and -D genes of Escherichia coli K-12 encode a glutathione transporter with the ATP-binding cassette. The transporter imports extracellular glutathione into the cytoplasm in an ATP-dependent manner. This transporter, along with γ-glutamyltranspeptidase, has an important role in E. coli growth with glutathione as a sole sulfur source.
Glutathione (γ-glutamylcysteinylglycine; GSH) plays a leading role in the protection of cells and organisms by reducing oxygen species and peroxides and acts against xenobiotics and drugs by the formation and excretion of glutathione S conjugates (16). Because of its protective effect on organs, glutathione has been dispensed to patients with hepatic diseases in Japan. Glutathione and its derivatives are moved in and out of cells by transporters of several types. The glutathione importers Na+-dependent transporter (10) and H+ symporter (9) are known, and the only glutathione transporters with the ATP-binding cassette identified so far are glutathione S conjugate exporters (4). As for microbial glutathione transporter, Saccharomyces cerevisiae YCF1 of the vacuolar membrane is known as a glutathione S-conjugate transporter (14), which also transports reduced glutathione into vacuoles (20), and yeast HGT1 of the plasma membrane is known as a membrane-potential-dependent glutathione importer across the plasma membrane (3). However, there has been no report on the identification of bacterial glutathione transporter.
It has been shown that Escherichia coli cells grown in aerobic conditions contain a large amount of glutathione (7). E. coli synthesizes glutathione by the sequential actions of γ-glutamylcysteine synthetase (the product of gshA) and glutathione synthetase (the product of gshB) as in other organisms (1). It excretes glutathione into the culture medium during the exponential phase and the concentration of glutathione in the culture medium reaches maximum in the early stationary phase (18, 26), but thereafter, it is hydrolyzed by γ-glutamyltranspeptidase (GGT) in the periplasm to liberate glutamic acid and cysteinylglycine (22, 26). Cysteinylglycine is taken up into the cytoplasm and then cleaved into cysteine and glycine by aminopeptidases A, B, and N and dipeptidase D to be utilized as a source of cysteine and glycine (25). We have suggested that this is the salvage pathway of cysteine for E. coli (23). It was also shown by other researchers in a mammalian cell line (8) and in yeast (15) that GGT catalyzes the initial step of the cleavage of extracellular glutathione for use as a source of cysteine and nitrogen. However, even in the case of a GGT-deficient strain of E. coli, the concentration of glutathione in the culture medium decreased gradually after prolonged incubation. This finding prompted us to search for a glutathione transporter which had never been reported in bacteria.
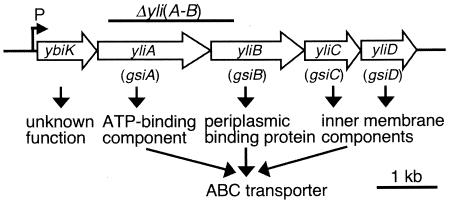
The ybiK gene was reported as a member of the cysB regulon and it was suggested to encode a protein involved in glutathione transport or metabolism (19), but its mechanism is still unclear. GenBank suggests that four genes, yliA, -B, -C, and -D, located downstream of ybiK, are transcribed with ybiK (Fig. 1). EcoCys (11) suggests that YliA, -B, -C, and -D are uncharacterized members of the ATP-binding cassette superfamily transporters. It suggests that yliA and -B encode the ATP-binding component and periplasmic binding protein, respectively, and that yliC and -D encode the plasma membrane components. From the above information, we speculated that YliA, -B, -C, and -D might encode the glutathione transporter.
FIG. 1.
The structure of the ybiK-yliABCD operon. The order, size, and products of the genes and the location of the promoter suggested by GenBank and EcoCys were diagrammed. The region of DNA deleted in our ΔyliAB mutation is shown by the bar above the genes. Gene names in parentheses are the new names proposed, after glutathione importer.
In this report, we present evidence indicating that yliA, -B, -C, and -D indeed encode a novel type of glutathione transporter.
MATERIALS AND METHODS
Bacterial strains, phage, plasmids, and oligonucleotides.
The bacterial strains, phage, plasmids, and oligonucleotides used in this study are listed in Table 1. Luria-Bertani (LB) broth (Miller) (17) purchased from Becton Dickinson was used regularly. M9 glucose medium (17) was used as a minimal medium. MgCl2 was used instead of MgSO4 when the effect of ΔyliAB mutation on the cell growth was measured. Ampicillin, tetracycline, and kanamycin were added at 100, 10, and 30 μg/ml, respectively, when appropriate. The bacteria were grown at 37°C with reciprocal shaking at 100 rpm.
TABLE 1.
Bacterial strains, phage, plasmids, and oligonucleotides used in this study
| Component | Description or sequence | Source or reference |
|---|---|---|
| Strains | ||
| JJP150 | F− φybiK-lacZ ΔybiK::KanrcysA nupC::Tn10 araD139 Δ(argF-lac)U169 rpsL150 deoC1 relA1 thiA ptsF25 ftb5301 | D. P. Clark (19) |
| MG1655 | F− prototrophic | C. A. Gross |
| SH639 | F− Δggt-2 | Spontaneous Tets mutant of SH703 selected on fusaric acid plate; laboratory stock |
| SH702 | F−zhg::Tn10 | 26 |
| SH703 | F− Δggt-2 zhg::Tn10 | 26 |
| SH1504 | F− Δggt-2 cysA nupC::Tn10 | SH639 × P1(JJP150); this work |
| SH1525 | SH1504 but ΔyliAB::Kanr | SH1504 × P1(SI26); this work |
| SH1527 | MG1655 but cysA nupC::Tn10 | MG1655 × P1(JJP150); this work |
| SH1535 | SH1527 but ΔyliAB::Kanr | SH1527 × P1(SI26); this work |
| SH1552 | pSI152/SI35 | This work |
| SH1554 | pSI151/SI35 | This work |
| SH1555 | pSI152/SI37 | This work |
| SH1617 | pSH1596/SI35 | This work |
| SH1618 | pSH1597/SI35 | This work |
| SH1619 | pSH1599/SI35 | This work |
| SH1620 | pSH1616/SI35 | This work |
| SI26 | TK251 but ΔyliAB::Kanr | This work |
| SI28 | SH703 but ΔyliAB::Kanr | SH703 × P1(SI26); this work |
| SI35 | SH703 but ΔyliAB | SI28 was transformed with pCP20 and grown at 37°C; this work |
| SI37 | pSH1391/SI35 | This work |
| SI49 | pSH1391/SH703 | This work |
| SI95 | TK251 but ΔgshA::Kanr | This work |
| SI96 | SI35 but ΔgshA::Kanr | SI35 × P1(SI95); this work |
| SI97 | SI35 but ΔgshA | SI96 was transformed with pCP20 and grown at 37°C; this work |
| SI99 | pSI80/SI97 | This work |
| SI100 | pSI83/SI97 | This work |
| SI101 | SH702 but ΔyliAB::Kanr | SH702 × P1(SI26); this work |
| SI102 | SH702 but ΔyliAB | SI101 was transformed with pCP20 |
| and grown at 37°C; this work | ||
| SI103 | pSH1391/SI102 | This work |
| SI104 | pSH1391/SH702 | This work |
| SI105 | SH703 but ΔgshA::Kanr | SH703 × P1(SI95); this work |
| SI106 | SH703 but ΔgshA | SI105 was transformed with pCP20 and grown at 37°C; this work |
| SI109 | pSI83/SI106 | This work |
| SI153 | pSI151/SI97 | This work |
| SI154 | pSI152/SI97 | This work |
| TK251 | pKD46/MG1655 | This work |
| Phage Kohara #208 | Y. Kohara (12) | |
| Plasmids | ||
| pCP20 | pSC101 replicon (ts) bla+cat+ Flp (λRp) cI857 | CGSCa (5) |
| pFK68 | ColEI replicon rop+ Tetr AmpsgshA+; 2.1-kb DNA fragment containing gshA was ligated with pBR322 cleaved with PvuI (blunt ended) and PstI (blunt ended) | This work |
| pKD13 | oriRγ bla+ FRT-Kanr-FRT | CGSC (5) |
| pKD46 | oriR101 replicon repA101tsaraC+gam+-bet+-exo+ (araBp) bla+ | CGSC (5) |
| pMC1871 | ColEI replicon rop+ Tetr ′lacZ | Pharmacia |
| pSH1391 | ColEI replicon rop+ Tets Amps KanrgshA+gshB+ | Laboratory stock |
| pSH1517 | ColEI replicon AmpryliA+; 2.3-kb CspI fragment (blunt ended) of Kohara #208-λDNA was ligated with pUC18 cleaved with HincII | This work |
| pSH1569 | pSI152 but the NcoI site was inserted 3 bp after the stop codon of yliD | This work |
| pSH1584 | pSH1569 but deleted DNA between the AatII (blunt-ended) site and NcoI site which locate upstream of ybiK | This work |
| pSH1596 | p15A replicon bla+ KansybiK+-yliA+yliB+yliC+yliD+-lacZ+; lacZ fragment amplified by P CR using oligonucleotides LacZ-fusion-up and LacZ-fusion-down, and pMC1871 as a template was ligated with pSH1584 cleaved with NcoI and HindIII | This work |
| pSH1597 | p15A replicon bla+ KansybiK+-yliB+yliC+yliD+-lacZ+; DNA region corresponds to the coding region of yliA looped out from pSH1596 using oligonucleotides delyliA and delyliA-comp | This work |
| pSH1599 | p15A replicon bla+ KansybiK+-yliA+C+D+-lacZ+; DNA region corresponds to the coding region of yliB looped out from pSH1596 using oligonucleotides delyliB and delyliB-comp | This work |
| pSH1616 | p15A replicon bla+ KansybiK+-yliA+yliB+yliD+-lacZ+; DNA region corresponds to the coding region of yliC looped out from pSH1596 using oligonucleotides delyliC and delyliC-comp | This work |
| pSI80 | p15A replicon bla+ KansybiK+-yliA+yliB+yliC+yliD+yliE+yliF+yliG+; 13.5-kb AatI (blunt-ended) fragment of Kohara #208-λDNA was ligated with pACYC177 cleaved with BamHI (blunt ended) and XhoI (blunt ended) | This work |
| pSI83 | p15A replicon bla+ Δkan; pACYC177 was cleaved with BamHI (blunt ended) and XhoI (blunt ended) and self-ligated | This work |
| pSI151 | p15A replicon bla+ KansybiK+-yliA+yliB+yliC+; pSI80 was cleaved with EcoRI (blunt ended) and HindIII (blunt ended) and self-ligated | This work |
| pSI152 | p15A replicon bla+ KansybiK+-yliA+yliB+yliC+yliD+; pSI80 was cleaved with SalI (blunt ended) and HindIII (blunt ended) and self-ligated | This work |
| Oligonucleotides | ||
| pKD13-1 | GTGTAGGCTGGAGCTGCTTC | |
| pKD13-4 | ATTCCGGGGATCCGTCGACC | |
| yliA-1 | AGTCTGCAACGCGGTGAGA | |
| yliA-2 | GCTGAGAACGGCTGTTTGAGG | |
| yliD-E NcoI | GGATCCGAAAATTAAAGGATAGTTACCATGGAATATTGCTTGAAAGGGTAATCACC | |
| yliD-E NcoI-comp | GGTGATTACCCTTTCAAGCAATATTCCATGGTAACTATCCTTTAATTTTCGGATCC | |
| LacZ-fusion-up | CCGCCGGCGCCATGGTCGTTTTACAACGTCGTGACTGGGb | |
| LacZ-fusion-down | GGGAAGCTTATTATTAATGATGATGATGATGATGTTTTTGACACCAGACCAACTGGTAATGG | |
| delyliA | GCCACACAGTGATGAATAACATTCAGGCGGAG | |
| delyliA-comp | CTCCGCCTGAATGTTATTCATCACTGTGTGGC | |
| delyliB | CAGGCGGAGAATAAAATGCTTAATTACGTTATCAAACG | |
| delyliB-comp | CGTTTGATAACGTAATTAAGCATTTTATTCTCCGCCTG | |
| delyliC | CAACGCAGGGAGTGGAATGCGACTATTTAACTGG | |
| delyliC-comp | CCAGTTAAATAGTCGCATTCCACTCCCTGCGTTG | |
| −286 | GGGCGATCGCCATTGCGTAAAACATCGCGC | |
| +1746 | GGGAACTGCAGGCGCTTCCATC | |
| gshA-1 | ATTGTGAAGATAGTTTACTGACTAGA | |
| gshA-2 | GGTAAAGCGTTACGCTATG |
CGSC, E. coli Genetic Stock Center.
Underlining shows the NcoI site at the initiation codon.
Strain and plasmid construction.
P1 transduction, DNA manipulation, and transformation were performed by the standard methods (17, 21). Gene disruption was performed according to the method of Datsenko and Wanner (5). Briefly, a ΔyliAB strain was constructed as follows. The DNA fragment of Kohara clone (12) #208 containing the yliA gene region was cloned onto pUC18 to obtain pSH1517. The FRT-Kanr-FRT fragment was amplified by PCR using oligonucleotides pKD13-1 and pKD13-4 as primers and pKD13 as a template with ExTaq DNA polymerase (Takara, Kyoto, Japan). The fragment was blunt ended with a blunting kit (Takara, Kyoto, Japan) and ligated with the 3.1-kb NruI and SacII (blunt-ended) fragment of pSH1517, which deleted most of the yliA gene and the N-terminal region of yliB (Fig. 1). The obtained plasmid was cleaved with EcoRI and HindIII, and the 1.8-kb fragment was used to transform strain TK251 by electroporation. Kanamycin-resistant transformant SI26 was obtained. The ΔyliAB::Kanr strain was transduced into the strains with appropriate backgrounds by the P1vir phage. The transductants were transformed with pCP20 at 30°C to eliminate the Kanr determinant. Then, pCP20 was cured by incubating the strains at 37°C. The deletion made on the genome was confirmed by colony PCR using oligonucleotides yliA-1 and yliA-2.
ΔgshA::Kanr and ΔgshA were constructed similarly. The DNA fragment containing the gshA gene region was amplified by PCR using oligonucleotides −286 and +1746 as primers and genomic DNA of E. coli as a template. The fragment was cleaved with PvuI and PstI and ligated with pBR322 cleaved with the same restriction endonucleases to obtain pFK68. The blunt-ended FRT-Kanr-FRT fragment obtained above was ligated with the 5.4-kb BglII (blunt-ended) and BssHII (blunt-ended) fragment of pFK68. The obtained plasmid was cleaved with AseI and ScaI, and the 2.6-kb fragment was used to transform strain TK251 by electroporation. Kanamycin-resistant transformant SI95 was obtained. ΔgshA::Kanr was moved into the strain SI35, and Kanr determinant was eliminated as described above. The deletion made on the genome was confirmed by colony PCR using oligonucleotides gshA-1 and gshA-2.
The plasmids with the yliA, -B, or -C gene deleted from pSH1596 were constructed as follows. The NcoI site was inserted 3 bp after the stop codon of yliD of pSI152 to obtain pSH1569 with yliD-E NcoI and yliD-E NcoI-comp as mutagenic primers by the QuikChange method (Stratagene) except KOD plus DNA polymerase (Toyobo, Osaka, Japan) was used. The DNA fragment between the AatII and NcoI sites, located upstream of ybiK, was deleted from pSH1569, and pSH1584 was made. The DNA fragment containing the coding region of the lacZ gene was amplified by PCR using oligonucleotides LacZ-fusion-up and LacZ-fusion-down and pMC1871 as a template. The fragment was cleaved with NcoI and HindIII and ligated with pSH1584 cleaved with the same restriction endonucleases to obtain pSH1596. Then, yliA gene was looped out from pSH1596 using oligonucleotides delyliA and delyliA-comp as mutagenic primers by the QuikChange method. The correctness of the DNA sequence of the mutated plasmid (pSH1597) was confirmed. DH5α was transformed with pSH1597, and the colonies of the transformant formed on the LB plate supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) were blue, as well as the DH5α transformed with pSH1596. This indicates that the ΔyliA deletion does not interfere with the transcription of the downstream genes of the operon, as intended. The yliB and -C genes were deleted from pSH1596 similarly. This ΔyliA deletion extends from the fifth base from the stop codon of ybiK to the base just before the stop codon of yliA. The ΔyliB deletion extends from the base just after the initiation codon of yliB to the initiation codon of yliC. The ΔyliC deletion extends from the initiation codon of yliC to the base just before the initiation codon of yliD.
Measurement of glutathione.
Reduced glutathione was measured using standard compounds (Sigma-Aldrich) with a high-pressure liquid chromatography (HPLC) instrument (model LC-9A; Shimadzu, Kyoto, Japan) equipped with a Shim-pack Amino-Na column and a fluorescence detector (model RF-535; Shimadzu), with o-phthalaldehyde as the detection reagent (24). Reduced and oxidized glutathione could be measured separately by this method. Total glutathione was measured with glutathione reductase (Sigma-Aldrich) by the method of Fahey et al. (6).
Transport assay.
Transport assay was performed as described previously (13) using [35S]glutathione (final concentration, 2 nM; 35.4 Tbq/mmol; PerkinElmer) except M9 glucose medium was used instead of M63 medium. When the effects of verapamil (Nacalai Tesque, Kyoto Japan) and carbonylcyanide-m-chlorophenylhydrazone (CCCP) (Nacalai Tesque, Kyoto Japan) were determined, cells were preincubated in the presence of these chemicals for 30 min at 37°C prior to the addition of labeled glutathione.
RESULTS
Effect of Δggt and ΔyliAB on the concentration of extracellular glutathione.
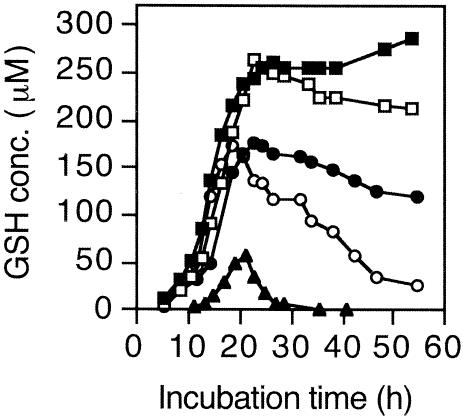
Since the wild-type E. coli accumulates only several μM glutathione at a maximum in the medium and it is difficult to measure such a low concentration of glutathione, the strains were transformed with a plasmid, pSH1391, which contains the gshA and gshB genes on pBR322, to overproduce glutathione-synthesizing enzymes. There was little difference in the growth among the strains used. The effects of Δggt and ΔyliAB on the concentration of extracellular glutathione were compared (Fig. 2). The concentration decreased after reaching the maximum during the early stationary phase when either ggt or yliAB was normal. That Δggt yliA+ yliB+ strain accumulates much more glutathione in the medium than the ggt+ ΔyliAB strain indicates that GGT is more effective in reducing the concentration of extracellular glutathione than the YliABCD transporter. On the other hand, the extracellular glutathione of the Δggt ΔyliAB strain gradually increased even during the stationary phase. When the Δggt ΔyliAB strain was complemented with pACYC177 containing the ybiK+-yliA+ yliB+ yliC+ yliD+ operon, the extracellular GSH was dramatically decreased.
FIG. 2.
Glutathione concentration of the culture media. Strains SI37 (Δggt ΔyliAB) (filled square), SI49 (Δggt) (open square), SH1555 (pACYC177::ybiK+-yliA+ yliB+ yliC+ yliD+/Δggt ΔyliAB) (filled triangle), SI103 (ΔyliAB) (filled circle), and SI104 (ggt+ yliA+ yliB+) (open circle) were grown in 100 ml minimal medium. At the times indicated, 2 ml of culture was subtracted. An optical density at 610 nm was measured using 1 ml of the 2-ml culture. Another 1 ml was centrifuged, and the concentration of glutathione of the culture fluid was measured with glutathione reductase.
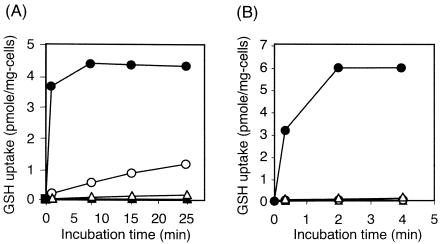
Transport assay of the YliABCD transporter.
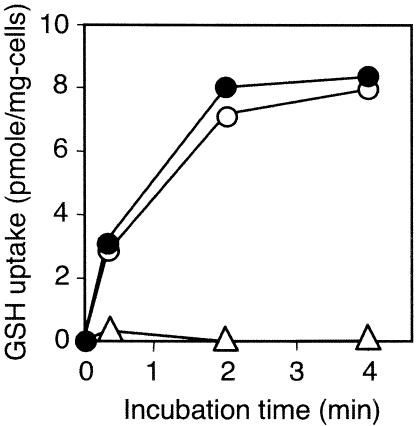
Transport assay was performed using [35S]glutathione and GGT-deficient derivatives (Fig. 3). The ΔyliAB strain transported practically no glutathione, while its yliA+ yliB+ derivative obviously transported glutathione. Moreover, ΔyliAB strain transformed with pACYC177 containing ybiK+-yliA+ yliB+ yliC+ yliD+ complemented the GSH transport phenotype. However, the same strain transformed with pACYC177 containing ybiK+-yliA+ yliB+ yliC+ did not complement the phenotype (Fig. 3A).
FIG. 3.
Glutathione uptake in a transporter assay. (A) Strains SI35 (Δggt ΔyliAB) (filled triangles), SH703 (Δggt) (open circles), SH1552 (pACYC177::ybiK+-yliA+ yliB+ yliC+ yliD+/Δggt ΔyliAB) (filled circles), and SH1554 (pACYC177::ybiK+-yliA+ yliB+ yliC+/Δggt ΔyliAB) (open triangles). (B) Strains SH1617 (pACYC177::ybiK+-yliA+ yliB+ yliC+ yliD+-lacZ+/Δggt ΔyliAB) (filled circles), SH1618 (pACYC177::ybiK+-yliB+ yliC+ yliD+-lacZ+/Δggt ΔyliAB) (open circles), SH1619 (pACYC177::ybiK+-yliA+ yliC+ yliD+-lacZ+/Δggt ΔyliAB) (filled triangles), and SH1620 (pACYC177::ybiK+-yliA+ yliB+ yliD+-lacZ+/Δggt ΔyliAB) (open triangles).
The plasmids containing ybiK+-yliA+ yliB+ yliC+ yliD+ but with yliA, -B, or -C deleted were also constructed. The ΔyliAB strain was transformed with these plasmids, and a transport assay was performed. Transport of glutathione was not observed when the operon deleted either yliA, -B, or -C (Fig. 3B).
Effect of ΔyliAB mutation on the intracellular concentration of glutathione.
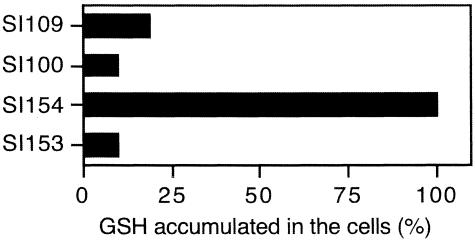
Glutathione-synthesis-deficient (ΔgshA) derivatives of the above strains were grown overnight in the minimal medium supplemented with or without 1 mM reduced glutathione. The cells were then opened by ultrasonication, and the amount of glutathione accumulated inside the cells was measured by HPLC (Fig. 4). All the glutathione found was in reduced form, and no oxidized form was observed. The amount of total glutathione measured with glutathione reductase agreed well with that of reduced glutathione found by HPLC (data not shown). When these four strains were grown in the minimal medium without glutathione, no detectable glutathione was found inside these strains. Although ΔyliAB mutation decreased the accumulation of glutathione inside the cells, nonnegligible accumulation of glutathione was observed even in the strain with ΔyliAB Δggt ΔgshA (strain SI100). The ΔyliAB mutation was complemented with pACYC177 containing ybiK+-yliA+ yliB+ yliC+ yliD+ (strain SI154), but not with pACYC177 containing ybiK+-yliA+ yliB+ yliC+ (strain SI153).
FIG.4.
Accumulation of glutathione in the cells grown in minimal medium supplemented with 1 mM glutathione. Strains SI100 (pACYC177/ΔgshA Δggt ΔyliAB), SI109 (pACYC177/ΔgshA Δggt), SI153 (pACYC177::ybiK+-yliA+ yliB+ yliC+/ΔgshA Δggt ΔyliAB), and SI154 (pACYC177::ybiK+-yliA+ yliB+ yliC+ yliD+/ΔgshA Δggt ΔyliAB) were grown in minimal medium supplemented with 1 mM reduced glutathione for 12 h. The amount of glutathione found in the cells was expressed as relative to that for strain SI154. Seventy-four nanomoles of glutathione per mg of cells was found in strain SI154.
Transport of glutathione by the YliABCD transporter depends on ATPase activity.
Effect of the ATPase inhibitor verapamil on glutathione transport by the YliABCD transporter was determined. Transport of glutathione by the YliABCD transporter was strongly inhibited in the presence of 10 mM verapamil (Fig. 5). Membrane potential inhibitor, CCCP, had no effect at 100 μM (data not shown).
FIG. 5.
Effect of verapamil on glutathione uptake. Glutathione uptake of strain SH1552 was measured in the absence of verapamil (filled circles) and in the presence of 1 mM (open circles) and 10 mM verapamil (open triangles).
Effect of the ΔyliAB mutation on cell growth.
The ability of the YliABCD transporter to utilize glutathione as a sole sulfur source was also investigated. The cysA gene encodes sulfate permease, and a cysA mutant cannot grow with SO42− in the medium and it is a cysteine auxotroph. The cysA Δggt (sulfate transport and GGT-deficient) strain grew weakly on minimal medium with 0.3 mM glutathione as a sole sulfur source (Fig. 6b, row 4), while almost no growth was observed for its ΔyliAB derivative on the same plate (Fig. 6b, row 3). The doubling times of the cysA, cysA ΔyliAB, cysA Δggt, and cysA ΔyliAB Δggt strains in minimal medium supplemented with 0.3 mM glutathione at 37°C were 1.7, 2.1, 3.2, and 12 h, respectively.
FIG. 6.
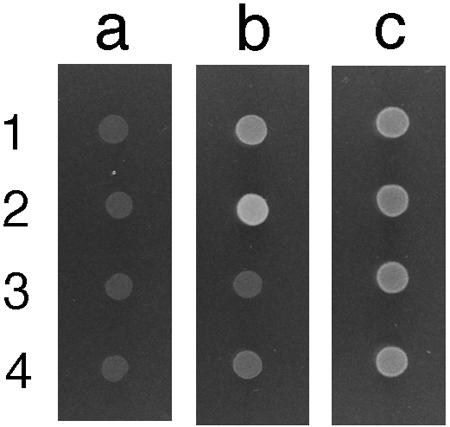
Growth of E. coli strains on a minimal medium plate supplemented with glutathione as the sole sulfur source. Strains were grown overnight in 5 ml LB medium, washed twice, and suspended with 5 ml of 1× M9 buffer. One μl of cell suspension was plotted on plates and grown overnight at 37°C. Strains 1 (SH1535; cysA ΔyliAB::Kanr), 2 (SH1527; cysA), 3 (SH1525; cysA Δggt ΔyliAB::Kanr), and 4 (SH1504; cysA Δggt) were grown on minimal medium supplemented with 0.05 mM thiamine without any sulfur source (column a) or with 0.3 mM glutathione (column b) or 0.3 mM cysteine (column c) as a sole sulfur source.
DISCUSSION
Our previous study of glutathione metabolism in E. coli indicated that catabolism of extracellular glutathione is initiated by the cleavage of its γ-glutamyl linkage by GGT (22, 26). However, the concentration of extracellular glutathione decreased gradually after prolonged incubation even when the ggt gene was deleted (Fig. 2, SI49) and the existence of a glutathione importer was suspected. As described in the introduction, we predicted that YliABCD is a glutathione importer. When both Δggt and ΔyliAB were deleted, the concentration of the extracellular glutathione did not decrease up to 55 h (Fig. 2, SI37). These results indicate that GGT and the YliABCD transporter are the two determinants that decrease the extracellular glutathione. Since yliA and -B are located upstream of yliC and -D and the promoter of the operon before ybiK (Fig. 1), the complementation test was performed using the plasmid containing whole operon.
A transport assay was performed using [35S]glutathione and GGT-deficient derivatives (Fig. 3). This is because GGT cleaves glutathione in the periplasm and cysteinylglycine uptake into the cytoplasm occurs. In fact, a ggt+ ΔyliAB strain took up a nonnegligible amount of 35S in a transport assay (data not shown). To avoid an overestimation of glutathione uptake by the transporter, GGT-deficient strains were used in this experiment. It was shown that uptake of 35S into the cells depends on the YliABCD transporter in GGT-deficient strains (Fig. 3A). The plasmid with yliA+ yliB+ yliC+ yliD+ could complement the ΔyliAB mutation, but the plasmid with yliA+ yliB+ yliC+ could not (Fig. 3A). This result strongly suggests that this ΔyliAB mutation has a polar effect on the downstream genes. Therefore, as shown in Fig. 3B, only one of the yliA, yliB, and yliC genes was carefully deleted with the intent of not causing a polar effect on the downstream genes. None of the deletions caused a polar effect, confirmed by the expression of the lacZ gene inserted just after the yliD gene. Our findings indicate that YliA, -B, -C, and -D are the components of the transporter because the transport activity was abolished if any one of them was deleted (Fig. 3A and B). The ybiK gene exists in front of yliABCD, and the possibility that they constitute an operon has been suggested (Fig. 1). Since the function of YbiK is not clear and its involvement in glutathione transport or metabolism has been suggested (19), there is a possibility that YbiK processes glutathione and then only a part of its molecule containing 35S is taken up into the cell. To deny this possibility and to show that the whole glutathione molecule is taken up by the YliABCD transporter, the concentration of glutathione accumulated inside glutathione-synthesis-deficient mutants grown in medium supplemented with glutathione was measured (Fig. 4). These results clearly show that the whole glutathione molecule was transported into the cell by the YliABCD transporter. Parry and Clark suggested the involvement of YbiK in glutathione transport or metabolism (19). Since they used strains with the ybiK::Kanr mutation, which has polar effect on yliA, -B, -C, and -D, their suggestion of the involvement of YbiK in glutathione transport might have been derived from this polar effect. A study on the role of YbiK is under way, and the results will be reported elsewhere.
Transport of glutathione was inhibited by verapamil, but not by CCCP. This indicates that this transport system depends on ATPase activity and not on membrane potential (Fig. 5).
To determine whether the transport of glutathione by the YliABCD transporter has physiological meaning, the effect of its absence on the growth of E. coli by using glutathione as a sole sulfur source was observed. The cysA mutant grew on a minimal medium plate supplemented with glutathione as a sole sulfur source (Fig. 6b, row 2). The growth of the cysA ΔyliAB strain (Fig. 6b, row 1) was obviously weaker than that of the cysA strain. The growth of the cysA Δggt strain was severely retarded (Fig. 6b, row 4), and almost no growth was observed for the cysA Δggt ΔyliAB strain on the same plate (Fig. 6b, row 3). The growth of cells in column c (minimum medium supplemented with cysteine as a sulfur source (Fig. 6c) was less than that of the cysA mutant on minimum medium supplemented with glutathione (Fig. 6b, row 2). We should mention that the addition of this much cysteine inhibits cell growth. The doubling time of these strains in the liquid minimal medium was compared, and the results indicated that both GGT and the YliABCD transporter are important in the growth of E. coli with glutathione as a sole sulfur source.
Although no detectable glutathione uptake was observed in the Δggt ΔyliAB strain by transport assay, there was some accumulation of glutathione inside the Δggt ΔyliAB ΔgshA strain grown in minimal medium supplemented with glutathione (Fig. 4, strain SI100). In fact, the cysA Δggt ΔyliAB strain could grow in minimal medium supplemented with glutathione as a sole sulfur source (Fig. 6b, row 3); however, its doubling time was extremely long. It is possibile that a nonspecific glutathione uptake system besides YliABCD and GGT exists in E. coli.
Boos and Lucht (2) reviewed the periplasmic binding-protein-dependent ABC transporters of E. coli, and they proposed a consensus sequence of the ATP-binding cassette subunits. The amino acid sequence of YliA conserved many of the consensus residues. YliA has repeats of Walker motif A-linker peptides-Walker motif B (Walker motif A, residues 55 through 63 and 363 through 371; linker peptides, residues 175 through 183 and 470 through 478; Walker motif B, residues 195 through 201 and 490 through 496). According to the MOTIF program (GenomeNet, Japan), YliB has a bacterial extracellular solute-binding protein family 5 signature (residues 76 through 98) and YliC and -D have binding-protein-dependent transport system inner membrane component signatures (residues 197 through 225 and 188 through 216, respectively). Also, the SignalP program (Technical University of Denmark) predicts that the first 26 amino acids of YliB constitute a signal peptide. The SOSUI program (Department of Biotechnology, Tokyo University of Agriculture and Technology) predicted that YliC and -D have six and seven transmembrane helices, respectively. All of these indicate that YliABCD composes an ATP-binding cassette superfamily transporter, YliA and -B being an ATP-binding component and a periplasmic binding protein, respectively, and YliC and -D being plasma membrane components. We propose the name gsi for these genes, after glutathione importer. yliA, -B, -C, and -D would be renamed gsiA, -B, -C, and -D, respectively.
This is the first report not only of bacterial glutathione transporter but also of a glutathione importer with an ATP-binding cassette among all organisms. The homology search suggests that Escherichia coli O157:H7, Shigella flexneri, Salmonella enterica serovar Typhi, and Salmonella enterica serovar Typhimurium have homologues. Our finding of a new glutathione importer with an ATP-binding cassette indicates that there is more diversity in the mechanism of glutathione transport across cell membranes than previously considered.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research no. 15580061 to H.S. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by a research grant from the Noda Institute for Scientific Research to H.S. T.K., S.I., and A.O. are supported by the 21st Century COE Program of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Apontowell, P., and W. Berends. 1975. Isolation and initial characterization of glutathione-deficient mutants of E. coli K12. Biochim. Biophys. Acta 399:1-9. [DOI] [PubMed] [Google Scholar]
- 2.Boos, W., and J. M. Lucht. 1996. Periplasmic binding protein-dependent ABC transporters, p. 1175-1209. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 3.Bourbouloux, A., P. Shahi, A. Chakladar, S. Delrot, and A. K. Bachhawat. 2000. Hgt1p, a high affinity glutathione transporter from yeast Saccharomyces cerevisiae. J. Biol. Chem. 275:13259-13265. [DOI] [PubMed] [Google Scholar]
- 4.Cole, S. P., G. Bhardwaj, J. H. Gerlach, J. E. Mackie, C. E. Grant, K. C. Almquist, A. J. Stewart, E. U. Kurz, A. M. Duncan, and R. G. Deeley. 1992. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258:1650-1654. [DOI] [PubMed] [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahey, R. C., S. Brody, and S. D. Mikolajczyk. 1975. Changes in the glutathione thiol-disulfide status of Neurospora crassa conidia during germination and aging. J. Bacteriol. 121:144-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahey, R. C., W. C. Brown, W. B. Adams, and M. B. Worsham. 1978. Occurrence of glutathione in bacteria. J. Bacteriol. 133:1126-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanigan, M. H., and W. A. Ricketts. 1993. Extracellular glutathione is a source of cysteine for cells that express γ-glutamyl transpeptidase. Biochemistry 32:6302-6306. [DOI] [PubMed] [Google Scholar]
- 9.Jamai, A., E. Martinoia, and S. Delrot. 1996. Characterization of glutathione uptake in broad bean leaf protoplasts. Plant Physiol. 111:1145-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannan, R., J. R. Yi, D. Tang, Y. Li, B. V. Zlokovic, and N. Kaplowitz. 1996. Evidence for the existence of a sodium-dependent glutathione (GSH) transporter. Expression of bovine brain capillary mRNA and size fractions in Xenopus laevis oocytes and dissociation from γ-glutamyltranspeptidase and facilitative GSH transporters. J. Biol. Chem. 271:9754-9758. [DOI] [PubMed] [Google Scholar]
- 11.Karp, P. D., M. Arnaud, J. Collado-Vides, J. Ingraham, I. T. Paulsen, and M. H. Saier, Jr. 2004. The E. coli EcoCyc database: no longer just a metabolic pathway database. ASM News 70:25-30. [Google Scholar]
- 12.Kohara, Y., K. Akiyama, and K. Isono. 1987. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell 50:495-508. [DOI] [PubMed] [Google Scholar]
- 13.Konayagi, T., T. Katayama, H. Suzuki, and H. Kumagai. 2004. Identification of the LIV-I/LS system as the third phenylalanine transporter in Escherichia coli K-12. J. Bacteriol. 186:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, Z.-S., M. Szczypka, Y.-P. Lu, D. J. Thiele, and P. A. Rea. 1996. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J. Biol. Chem. 271:6509-6517. [DOI] [PubMed] [Google Scholar]
- 15.Mehdi, K., and M. J. Penninckx. 1997. An important role for glutathione and γ-glutamyltranspeptidase in the supply of growth requirements during nitrogen starvation of the yeast Saccharomyces cerevisiae. Microbiology 143:1885-1889. [DOI] [PubMed] [Google Scholar]
- 16.Meister, A. 1989. A brief history of glutathione and a survey of its metabolism and functions, p. 1-48. In D. Dolphin, R. Poulson, and O. Avramovic (ed.), Glutathione, chemical, biochemical, and medical aspects. John Wiley and Sons, New York, N.Y.
- 17.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 18.Owens, R. A., and P. E. Hartman. 1986. Export of glutathione by some widely used Salmonella typhimurium and Escherichia coli strains. J. Bacteriol. 168:109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parry, J., and D. P. Clark. 2002. Identification of a CysB-regulated gene involved in glutathione transport in Escherichia coli. FEMS Microbiol. Lett. 209:81-85. [DOI] [PubMed] [Google Scholar]
- 20.Rebbeor, J. F., G. C. Connolly, M. E. Dumont, and N. Ballatori. 1998. ATP-dependent transport of reduced glutathione on YCF1, the yeast orthologue of mammalian multidrug resistance associated protein. J. Biol. Chem. 273:33449-33454. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Suzuki, H., W. Hashimoto, and H. Kumagai. 1993. Escherichia coli K-12 can utilize an exogenous γ-glutamyl peptide as an amino acid source, for which γ-glutamyltranspeptidase is essential. J. Bacteriol. 175:6038-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki, H., W. Hashimoto, and H. Kumagai. 1999. Glutathione metabolism in Escherichia coli. J. Mol. Catal. B 6:175-184. [Google Scholar]
- 24.Suzuki, H., S. Izuka, H. Minami, N. Miyakawa, S. Ishihara, and H. Kumagai. 2003. Use of bacterial γ-glutamyltranspeptidase for enzymatic synthesis of γ-d-glutamyl compounds. Appl. Environ. Microbiol. 69:6399-6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki, H., S. Kamatani, E.-S. Kim, and H. Kumagai. 2001. Aminopeptidases A, B, and N and dipeptidase D are the four cysteinylglycinases of Escherichia coli K-12. J. Bacteriol. 183:1489-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki, H., H. Kumagai, and T. Tochikura. 1987. Isolation, genetic mapping, and characterization of Escherichia coli K-12 mutants lacking γ-glutamyltranspeptidase. J. Bacteriol. 169:3926-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]