Figure 1.
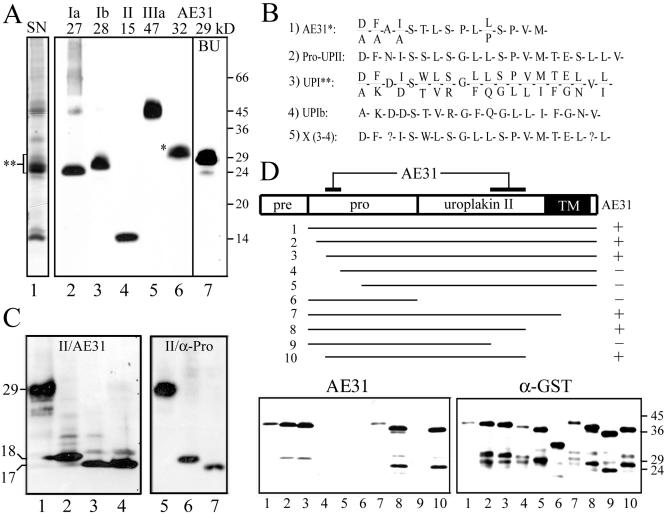
The AE31 mAb recognizes a composite epitope of pro-UPII. (A) Total proteins of purified bovine urothelial plaques (AUM; lanes 1–6) or cultured bovine urothelial (BU) cells (lane 7) were separated by SDS-PAGE and stained with (lane 1) silver nitrate (SN); or immunoblotted using rabbit antibodies to (2) UPIa, (3) UPIb, or (4) UPII, or (5) mouse AU1 mAb to UPIIIa, or (6 and 7) mouse AE31 mAb. Note that AE31 recognized a 32-kDa protein in the AUM and a 29-kDa protein in cultured BU cells. (B) N-terminal sequence of the bovine AE31 antigen. Listed are the N-terminal sequences of (1) the 32-kDa AE31 antigen immunoaffinity-purified from the Triton X-100 solubilized total urothelial plaque proteins; (2) the cDNA-deduced prosequence of pro-UPII (Lin et al., 1994); (3) the SDS-PAGE-purified, 27–32-kDa proteins of the bovine AUM (double-asterisked region in lane 1 of A); (4) the cDNA-deduced UPIb sequence (Yu et al., 1994); and (5) a hypothetical protein (X)— deduced by subtracting the UPIb sequence shown in (4) from the mixed sequence shown in (3) (described as “UPIc” in Yu et al., 1994). Note that the N-terminal sequence of the AE31 antigen was almost identical to that of pro-UPII and X. (C) Immunoblotting of total proteins of UPII cDNA-transfected COS-1 cells using (lanes 1–4) the AE31 mAb or (4–7) a rabbit antiserum against the prosequence portion of pro-UPII. Samples in lanes (2) and (6) were endo H-treated; (3) and (7) endo F-treated; cells of lane (4) were treated with tunicamycin (6.4 μg/ml). Note that both AE31 and the anti-prosequence antibody recognized the same 29-kDa glycoprotein that became 18- and 17-kDa after endo H and endo F treatments, respectively. (D) Mapping of the AE31 epitope in pro-UPII. Lanes 1–10 showed the immunoblotting of 10 pro-UPII fragments (in the form of GST-(truncated pro-UPII) fusion proteins; amino acids –59 to +100, –55 to +100, –50 to +100, –40 to +100, –30 to +100, –59 to –1, –59 to +80, –59 to +60, –59 to +40, and –50 to +60) using the AE31 mAb (lower left) or a mAb against the GST protein (lower right; as a control). The highest MW bands recognized by both AE31 and anti-GST antibodies represent the intact fusion proteins (no. 1–10); lower MW bands are degradation products. Note that AE31-reaction with pro-UPII required the simultaneous presence of two distant domains (amino acid positions –50 to –40 in the prosequence and positions +40 to +60 in the mature UPII), suggesting that pro-UPII assumed a folded, hairpinlike structure that was stable in the SDS-PAGE condition.