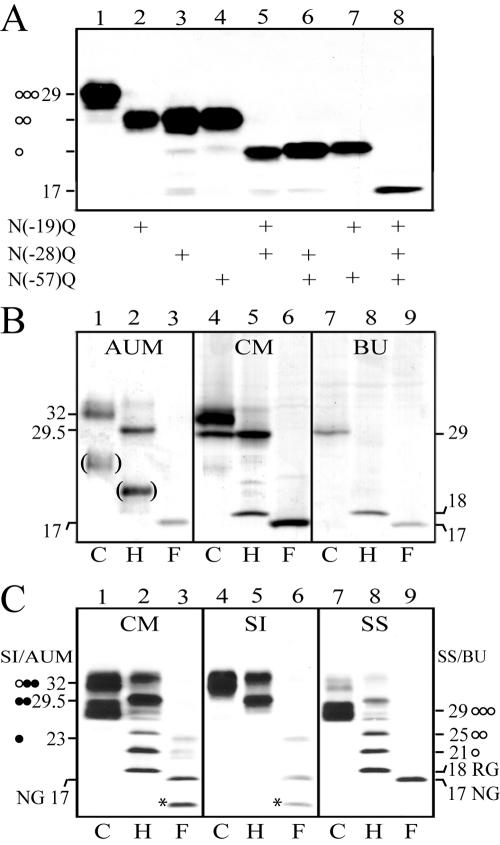
Figure 5.
Differentiation-dependent glycosylation of pro-UPII. (A) COS-1 cells were transfected with wild-type UPII cDNA (lane 1) or with UPII cDNAs mutating one (lanes 2–4), two (lanes 5–7), or all three (lane 8) of the potential N-glycosylation sites (Asn at positions –19, –28 and –57 mutated to Gln) in the prosequence region, and the cell lysates were immunoblotted using the AE31 mAb. Note that the mutation of each site led to the loss of an almost identical size reduction suggesting that all three sites harbored about the same amounts of sugar. (B) Proteins of bovine urothelial plaques (AUM), crude membranes (CM) of normal bovine urothelium, and cultured BU cells (total lysates) were incubated with the deglycosylation buffer (C or control; lanes 1, 4, and 7), endo H (H; lanes 2, 5, and 8), or endo F (F; lanes 3, 6, and 9) at 37°C for 16 h (for complete deglycosylation), and immunoblotted using the AE31 mAb. Note the presence of the 32-kDa AE31 antigen in the AUM, the 29-kDa antigen in cultured BU cells, and both of the two bands in the crude membranes (CM) of normal bovine urothelium. (C) Crude membranes (CM) of bovine urothelium were separated into the Sarkosylinsoluble (SI) AUM and Sarkosyl-soluble (SS) membrane protein fractions. All these proteins were incubated with the deglycosylation buffer (C; lanes 1, 4, and 7), endo H (H; lanes 2, 5, and 8), or endo F (F; lanes 3, 6, and 9) at 25°C for 6 h (for partial deglycosylation), and immunoblotted using the AE31 mAb. Note that the 32-kDa pro-UPII of the CM was Sarkosyl-insoluble (lane 4), whereas the 29-kDa species was Sarkosyl-soluble (lane 7). The numbers indicate molecular weights; the open and closed circles denote high-mannose and complex glycans, respectively; and RG/18-kDa and NG/17-kDa denote the pro-UPII harboring the residual glycan (N-acetylglucosamines) and no glycan, respectively. The bracketed bands in B (showing samples that have been subjected to prolonged deglycosylation reactions) mostly likely represented degradation products; the asterisked ∼14-kDa degradation product in C was only occasionally observed. These results indicate that the 29-kDa pro-UPII harbors three high-mannose glycans (as indicated by the 25-, 21-, and 18-kDa intermediates), whereas the 32-kDa pro-UPII harbors one high-mannose and two complex glycans (29.5-, 23-, and 17-kDa intermediates; it is unknown, however, which two sites harbor the complex glycans).