Figure 4.
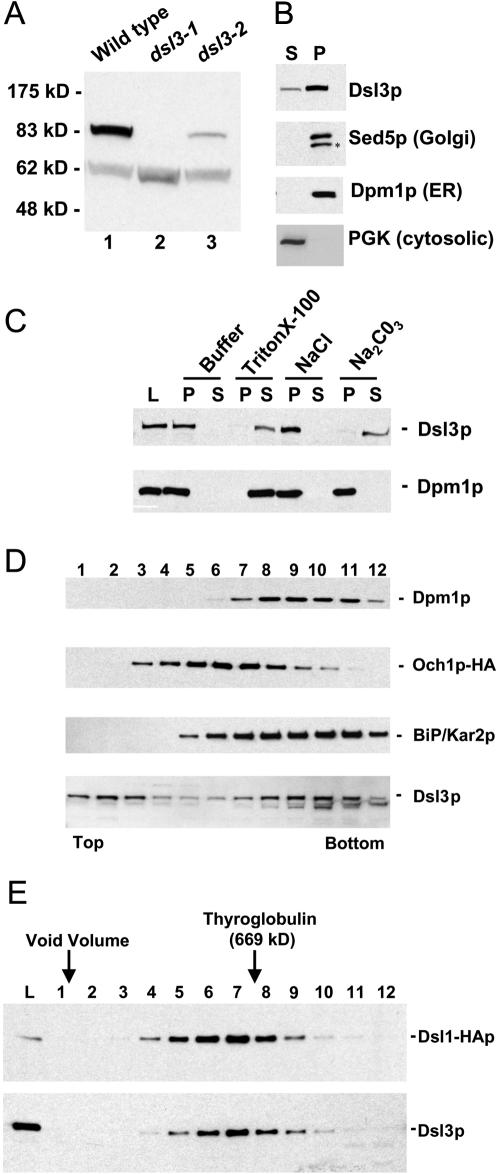
Dsl3p is a peripheral membrane protein that cofractionates with ER proteins and is in the same large complex as Dsl1p. (A) Characterization of the anti-Dsl3p antibody. Total yeast lysates prepared from a wild-type strain (RSY255) or a dsl3Δ strain bearing dsl3-1 (GWY601) or the dsl3-2 (GWY603) were separated by SDS-12% PAGE and immunoblotted with antibodies against Dsl3p. The migration of molecular mass standards (New England Biolabs, Beverly, MA) is shown on the left. (B) Subcellular fractionation of Dsl3p. A yeast lysate was centrifuged at 175,000 × g and separated into supernatant (S) and pellet (P) fractionation, which were resolved by SDS-12% PAGE and immunoblotted with antibodies against Dsl3p, Sed5p, Dpm1p, or PGK, as indicated. The asterisk indicates a proteolytic product of Sed5p. (C) Dsl3p is a tightly bound peripheral membrane protein. Total yeast membranes were isolated on a step gradient and incubated for 45 min in either buffer, 1% Triton X-100, 1 M NaCl, or 100 mM Na2CO3, pH 11. Reaction mixtures were separated into supernatant (S) and pellet (P) fractions by centrifugation at 175,000 × g for 60 min and analyzed by SDS-12% PAGE and immunoblotting with antibodies against Dsl3p (top panel) or Dpm1p (bottom panel). (D) Dsl3p cofractionates with BiP/Kar2p and Dpm1p and does not significantly overlap with Och1p-HA by buoyant density centrifugation. Membranes from an Och1p-HA-expressing strain (RSY255 bearing pSV66) were separated by buoyant density centrifugation. Gradient fractions were analyzed by immunoblotting with anti-Dsl3p, anti-BiP/Kar2p, anti-Dpm1p, and anti-HA antibodies as indicated. Fractions obtained from the top and bottom of the gradient are indicated. (E) Dsl3p is present in a large complex with Dsl1p. Size exclusion chromatography of Triton X-100-extracted membranes was followed by immunoblotting fractions with anti-Dsl1p and anti-Dsl3p antibodies.