Figure 8.
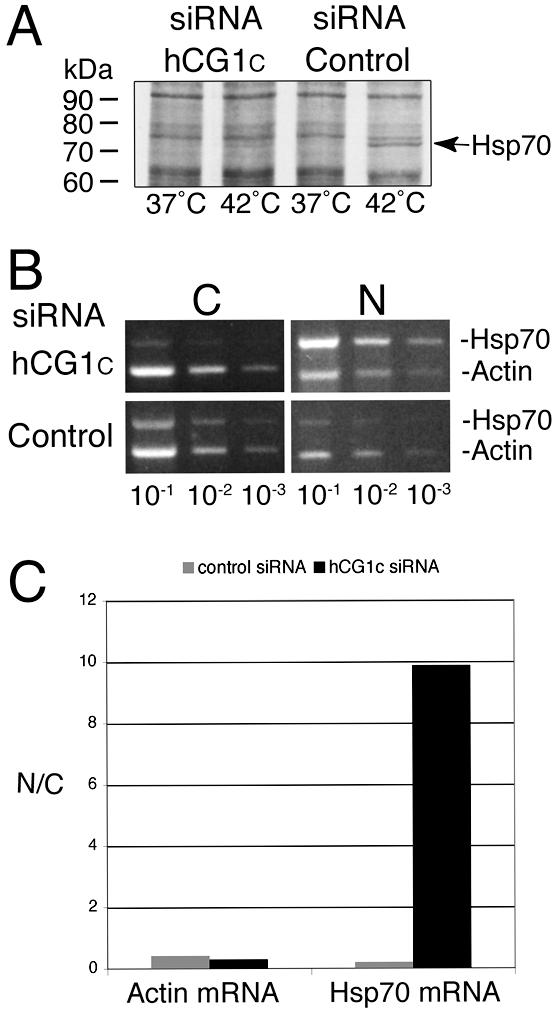
siRNA depletion of hCG1 inhibits Hsp70 protein production and nuclear export of Hsp70 mRNA. (A) Assessment of Hsp70 heat-shock protein expression levels in siRNA transfected cells. Forty-eight hours after transfection with 30 nM of hCG1C siRNAs or control siRNAs, HeLa cells were heat-shocked for 2 h in the presence of [35S]methionine. Total cell protein was subsequently extracted in SDS-sample buffer and resolved by SDS-PAGE followed by autoradiography. (B) Semiquantitative analysis of actin and Hsp70 mRNA distribution in subcellular fractions of siRNA treated HeLa cells after heat shock. After treatment with siRNA pools, cells were heat-shocked at 42°C for 2 h and total RNA from nuclear and cytoplasmic fractions was isolated and reverse-transcribed with actin- and Hsp70-specific primers. Serial dilutions were made and subsaturating PCR-based amplifications were performed. The products were separated on agarose gels and stained with ethidium bromide. (N, nuclear fraction; C, cytoplasmic fraction). (C) Quantitative analysis of the representative fractionation experiment in B. Bands corresponding to Hsp70 and actin PCR products in the linear dilution range (10-2 dilution) were quantified (see Materials and Methods), and the respective nuclear/cytoplasmic ratio was plotted as a bar graph.
