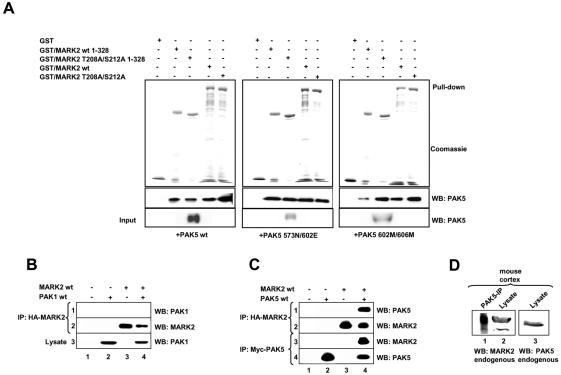
Figure 2.
Interaction of PAK5 with MARK2 determined by GST pulldown assay and coprecipitation. (A) His-tagged PAK5 expressed in Sf9 cells (wt 75 nM, active 30 nM, or inactive mutant 10 nM) was pulled down with equal amounts (1.5 μM) of different bacterially purified GST-MARK2 constructs. The pulldown fractions were analyzed by SDS-PAGE followed by Coomassie staining (GST-constructs) or immunoblotting with anti-PAK5 antibody. Quantification shows that typically 2-3% of PAK5 was pulled down. (B and C) HEK293 cells were transfected with plasmids encoding CFP-PAK1 (B), myc-tagged PAK5 (C) and HA-tagged MARK2 either singly (lanes 2 and 3) or in combination (lanes 4). Empty pEU vector (lane 1) was used to make the total amount of transfected DNA equivalent. Cell lysates were immunoprecipitated with anti-HA antibody (B, row 2, and C, row 2 and 3) and immunoblotted either with anti-PAK1 antibody (B, row 1) or anti-myc antibody (C, row 1). Expression of PAK1 and myc-tagged PAK5 was analyzed by immunoblotting with anti-PAK1 antibody (B, row 3) or anti-myc-antibody (C, row 3). (D) The cortex of an adult mouse was lysed and endogenous PAK5 was immunoprecipitated with anti-PAK5 antibody. The same probe was immunoblotted with anti-MARK2 antibody (lane 1). Endogenous expression of both proteins in the lysate was analyzed with the corresponding antibodies (lanes 2 and 3). IP, immunoprecipitation; WB, Western blotting.