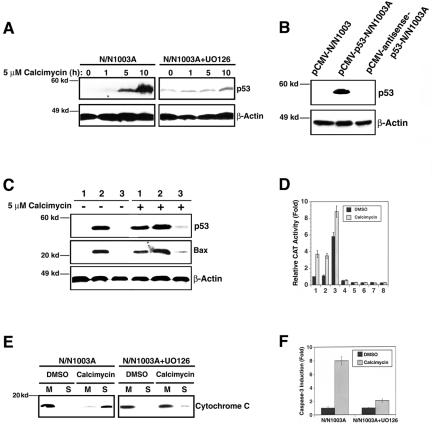
Figure 3.
Analysis of the apoptotic pathway downstream of ERK1/2 activation. (A) Western blot analysis to show that calcimycin induces up-regulation of p53 (left) and that such up-regulation is abrogated when ERK1/2 activation is inhibited (right). N/N1003A cells were grown to 100% confluence and then either pretreated with 50 μM UO126 (right) or 0.01% DMSO (left) for 6 h followed by treatment with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Then, total proteins were isolated, resolved in 10% SDS-PAGE and analyzed with antibodies against p53 and β-actin as described previously (Li et al., 1998). Note that the UO126 pretreatment enhanced the background p53 expression but abrogated its up-regulation by calcimycin. (B) Western blot analysis to show the different p53 levels at pCMV vector-transfected N/N1003A cells, pCMV-p53 transfected N/N1003A cells, and pCMV-antisense-p53-transfected N/N1003A cells. The three types of cells were grown to 100% confluence, and then total proteins were isolated, resolved in 10% SDS-PAGE, and analyzed with antibodies against p53 and β-actin as described in Figure 3A. (C) Western blot analysis of p53 and Bax levels in the vector-transfected cells (1), p53-transfected cells (2), and antisense-p53-transfected cells (3) under treatment of 0.01% DMSO (-5 μM calcimycin) or 5 μM calcimycin (+5 μM calcimycin). Western blot was conducted with antibodies against p53, Bax, and βactin as described previously (Li et al., 1998). Both exogenous and endogenous p53 has the same molecular weight. (D) p53 positively regulates Bax in RLECs. Columns 1-4, the results from the reporter gene with wild-type p53 binding site (pBax-CAT), and columns 5-8, the results from the reporter gene with mutant p53 binding sites [pBax(m)-CAT]. Columns 1 and 5, parental N/N1003A cells; columns 2 and 6, vector-transfected cells (pCMV-N/N1003A), columns 3 and 7, p53-transfected cells (pCMV-p53-N/N1003A), and columns 4 and 8, antisense-p53-transfected cells (pCMV-antisense-p53-N/N1003A). pBax-CAT or pBax(m)-CAT and pRSV-β-Gal were cotransfected into the four types of cells. Twenty-four hours after transfection, the four types of cells were treated with either 0.01% dimethyl sulfoxide or 5 μM calcimycin for 6 h. Thereafter, the different cell samples were harvested for assay of both β-Gal and CAT activity as described previously (Xiang et al., 2002; Feng et al., 2004). As controls, the Bax-CAT with p53 site mutated was also cotransfected with pRSV-β-Gal. The results of CAT activity generated from this mutant construct was close to background and displayed little difference in different cell lines with or without calcimycin treatment (columns 5-8). (E) UO126 prevents release of cytochrome c. N/N1003A cells were grown to 100% confluence and then pretreated with either 50 μM UO126 (right) or 0.01% DMSO (left) for 6 h followed by treatment with 5 μM calcimycin (calcimycin) or 0.01% DMSO (DMSO) for 12 h in MEM. Then, the treated cells were collected for isolation of mitochondria (M) and soluble fraction (S). Total proteins in both mitochondria and soluble fraction were resolved in 12% SDS-PAGE and analyzed with an antibody against cytochrome c as described previously (Mao et al., 2004). (F) UO126 prevents activation of caspase-3. N/N1003A grown to 100% confluence were pretreated with either 50 μM UO126 or 0.01% DMSO for 6 h followed by treatment with 5 μM calcimycin (calcimycin) or 0.01% DMSO (DMSO) for 12 h in MEM. At the end of the treatment, each sample was collected for caspase-3 activity assays as described previously (Li et al., 2001; Mao et al., 2001, 2004).