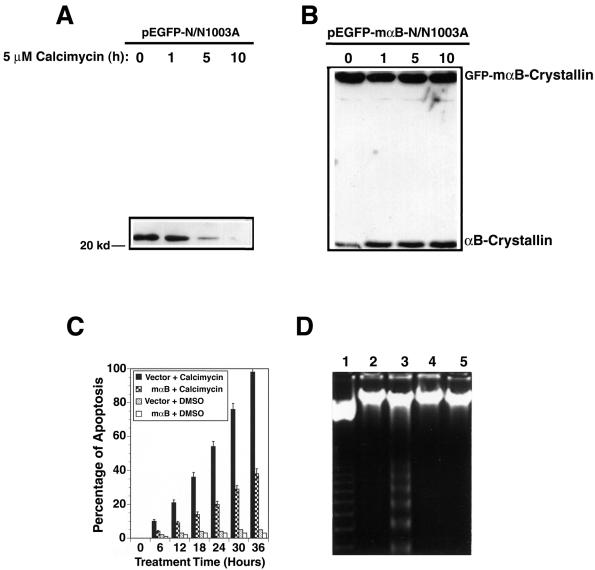
Figure 4.
αB-crystallin prevents calcimycin-induced apoptosis. (A) Calcimycin-induced degradation of the endogenous αB-crystallin. The vector-transfected cells (pEGFP-N/N1003A) were grown to 100% confluence, and subsequently treated with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Then, total proteins were isolated, resolved in 10% SDS-PAGE, and analyzed with antibody against αB-crystallin as described previously (Mao et al., 2001, 2004). Note that the endogenous αB-crystallin became gradually degraded after treatment by 5 μM calcimycin. (B) Overexpression of the fusion protein of GFP and mouse αB-crystallin not only provided resistance to the exogenous fusion protein but also stabilized the endogenous αB-crystallin. The GFP-αB-crystallin-transfected N/N1003A cells were grown to 100% confluence, and subsequently treated with 5 μM calcimycin for 0, 1, 5, or 10 h in MEM. Western blot analysis was conducted as described in Figure 4A. (C) Apoptosis assays. Vector-transfected cells (vector), and mouse αB-crystallin-transfected cells (mαB) were grown to 100% confluence in MEM with 400 μg/ml G418. Then, they were treated with 5 μM calcimycin (calcimycin) or 0.01% DMSO (DMSO) for 0-36 h. The percentage of cell death was determined as described previously (Li et al., 1998). (D) DNA fragmentation assay. The vector-transfected cells (lanes 2 and 3), and mouse αB-crystallin-transfected cells (lanes 4 and 5) were treated with 0.01% DMSO (lanes 2 and 4) or 5 μM calcimycin (lanes 3 and 5) for 18 h. These cells were collected for extraction of genomic DNAs which were processed as described in Figure 1B. The 123 base pairs DNA marker (Invitrogen) is shown in lane 1.