Abstract
FOXP2, the first gene to have been implicated in a developmental communication disorder, offers a unique entry point into neuromolecular mechanisms influencing human speech and language acquisition. In multiple members of the well-studied KE family, a heterozygous missense mutation in FOXP2 causes problems in sequencing muscle movements required for articulating speech (developmental verbal dyspraxia), accompanied by wider deficits in linguistic and grammatical processing. Chromosomal rearrangements involving this locus have also been identified. Analyses of FOXP2 coding sequence in typical forms of specific language impairment (SLI), autism, and dyslexia have not uncovered any etiological variants. However, no previous study has performed mutation screening of children with a primary diagnosis of verbal dyspraxia, the most overt feature of the disorder in affected members of the KE family. Here, we report investigations of the entire coding region of FOXP2, including alternatively spliced exons, in 49 probands affected with verbal dyspraxia. We detected variants that alter FOXP2 protein sequence in three probands. One such variant is a heterozygous nonsense mutation that yields a dramatically truncated protein product and cosegregates with speech and language difficulties in the proband, his affected sibling, and their mother. Our discovery of the first nonsense mutation in FOXP2 now opens the door for detailed investigations of neurodevelopment in people carrying different etiological variants of the gene. This endeavor will be crucial for gaining insight into the role of FOXP2 in human cognition.
The FOXP2 gene (MIM 605317) has been implicated in a severe form of speech and language disorder (SPCH1 [MIM 602081]) found in a large multigenerational pedigree and in an independent chromosome translocation case (Lai et al. 2001). This discovery has had an impact on a range of fields, including genetics, neuroscience, clinical neurology, and evolutionary anthropology (for reviews, see Fisher et al. 2003; Marcus and Fisher 2003; Vargha-Khadem et al. 2005). FOXP2, located in band 7q31, encodes a transcription factor, containing polyglutamine tracts, a zinc finger, a leucine zipper motif, and a forkhead-box DNA-binding domain (Lai et al. 2001; Wang et al. 2003), and it is likely to regulate gene expression in defined areas of developing lung, cardiovascular, intestinal, and neural tissue (Shu et al. 2001; Lai et al. 2003). The available expression data are compatible with a conserved role (or roles) for this gene in regulating development of neural circuitry underlying motor control and sensory-motor integration in mammals and birds (Ferland et al. 2003; Lai et al. 2003; Takahashi et al. 2003; Haesler et al. 2004; Teramitsu et al. 2004). Notwithstanding the high conservation of FoxP2 gene sequences and expression patterns in the different vertebrate species studied thus far, evolutionary analyses have demonstrated that the human version of the gene has been subject to positive selection within the past 200,000 years (Enard et al. 2002; Zhang et al. 2002). Thus, the relevant neurogenetic pathways may have undergone human-specific modifications at a time when spoken language was emerging (Enard et al. 2002; Zhang et al. 2002), although the precise nature of the putative mechanisms has yet to be determined.
FOXP2 represents a unique molecular entry point into the investigation of neuronal processes involved in speech and language. However, there has been considerable debate over whether sequence variation in this gene might have significance beyond the unusual case of the KE family. In this three-generation pedigree, a heterozygous missense mutation (Lai et al. 2001) cosegregates with deficits in sequencing the complex, coordinated orofacial movements required for speech (verbal dyspraxia), accompanied by a wide range of linguistic and grammatical deficits (Vargha-Khadem et al. 1998; Watkins et al. 2002). The mutation results in an arginine-to-histidine substitution (R553H) at a highly conserved residue within the DNA-binding domain (Lai et al. 2001). In vitro studies of the corresponding substitution in another forkhead-box protein (R127H in human FOXC1) suggest that the change can dramatically interfere with DNA-binding and transactivation capacity (Saleem et al. 2003), although this has not yet been demonstrated for FOXP2 itself. The FOXP2 locus has also been found to be directly disrupted in an independent case of speech and language disorder (CS) associated with a de novo balanced translocation involving chromosomes 7 and 5 (Lai et al. 2001).
Following identification of the KE family mutation and CS translocation, many studies have carried out targeted linkage/association analyses of the 7q region or mutation screening of FOXP2 in children with language-related disorders, including specific language impairment (SLI [MIM 606711]), autism (MIM 608636), and dyslexia (MIM 604254) (e.g., Newbury et al. 2002; Wassink et al. 2002; Gauthier et al. 2003; Kaminen et al. 2003; O’Brien et al. 2003). Despite such efforts, the missense mutation of the KE family remains the single reported case of an unambiguous etiological variant in FOXP2 coding sequence in the literature. This places limits on what can be learned about the influences of FOXP2 on human brain development, since, up to this point, all the relevant neuropsychological and neuroimaging investigations have necessarily been limited to just this one allelic variant (Vargha-Khadem et al. 1998; Watkins et al. 2002; Belton et al. 2003; Liégeois et al. 2003) or to gross chromosomal rearrangements that may involve effects of multiple additional genes (Liégeois et al. 2001).
Our current investigation was driven by the observation that, to date, no study had fully examined the sequence of FOXP2 in children with a phenotypic profile which closely corresponds to that found in the KE family (or to that found in cases of chromosomal abnormality involving FOXP2). Although a broad range of language-related deficits are evident in these individuals, the most overt symptom of their disorder is a severe developmental verbal dyspraxia, and it is this feature that provides the most reliable indicator of affection status (Vargha-Khadem et al. 1998; Liégeois et al. 2001; Watkins et al. 2002). Notably, previous screening studies of the entire FOXP2 ORF have focused on individuals affected with typical SLI, autism, or dyslexia (Newbury et al. 2002; Wassink et al. 2002; Gauthier et al. 2003; Kaminen et al. 2003). Verbal dyspraxia might indeed be present in a subset of such children, but it is not generally considered to be a core feature for any of these developmental disorders (World Health Organization 1993; American Psychiatric Association 1994) and may represent the defining symptom of a distinctive clinical entity (or entities) (Lewis et al. 2004). Given that FOXP2 was the first known case of a gene to be implicated in impaired speech and language acquisition, it was appropriate for these previous investigations to assess its possible involvement in a variety of language-related disorders. Nevertheless, for the present study, we hypothesized that we might maximize the possibility of detecting further etiological variants by screening, for the first time, a panel of patients with a primary diagnosis of verbal dyspraxia.
After the publication by Lai et al. (2001), our department received many referrals of cases with unexplained speech and language deficits, requesting testing for FOXP2 abnormalities. To more closely match the phenotype of the KE family, patients were selected for FOXP2 screening only if they fulfilled the following criteria: presence of speech articulation problems diagnosed by a clinician (e.g., a pediatrician or neurologist), normal karyotype, absence of mental retardation and congenital abnormalities, normal hearing, and no other medical/genetic diagnosis. The study was approved by the Oxfordshire Clinical Research Ethics Committee. Patients were enrolled sequentially, with no preference given to those with a family history of language disorder. The referring doctors and parents filled in a questionnaire for the study, which contained confirmation of the phenotype and provided results of the karyotype, speech examination report, and family history. DNA samples were collected from 49 probands, of whom 10 had one (or more) affected siblings whose samples were also included in the study. All affected cases were white, were within the 4–12-year-old age group (except for one pair of siblings aged 30 and 33 years), and resided in Europe, Australia, or the United States.
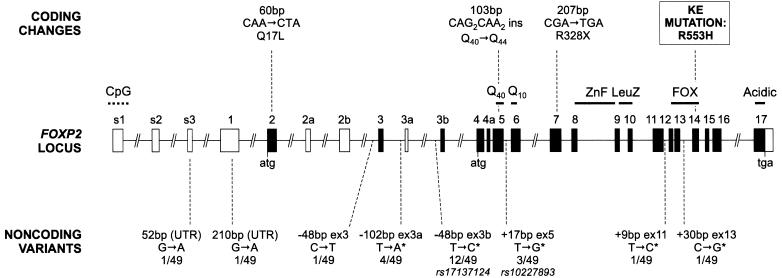
We conducted mutation screening across all FOXP2 exons identified by Lai et al. (2001) via denaturing high-performance liquid chromatography (DHPLC), followed by direct sequencing of fragments showing variant elution patterns, using identical protocols and primers to those described elsewhere by Newbury et al. (2002). We similarly analyzed additional alternatively spliced and 5′ UTR exons reported by Bruce and Margolis (2002) (see table A1 for sequences of all primers). All assays spanned entire exons, plus short stretches of flanking intronic sequence. Screening of our cohort of 49 probands revealed two 5′ UTR variants and six intronic changes (fig. 1). Five of these changes correspond to polymorphisms reported in earlier studies of FOXP2 (Newbury et al. 2002), and two overlap with SNPs that are present in public databases (rs17137124 and rs10227893). In support of the sensitivity of our DHPLC-based approach, the public databases contain no other validated SNPs in the regions of FOXP2 investigated in this study.
Figure 1.
Schematic of the human FOXP2 locus, which spans >600 kb of genomic DNA, showing sequence variants identified in subjects with verbal dyspraxia. Black shading indicates translated exons; “atg” and “tga” denote positions of initiation and termination codons. Known domains encoded by exons include polyglutamine tracts (Q40 and Q10), a zinc-finger motif (ZnF), a leucine zipper (LeuZ), the forkhead domain (FOX), and an acidic C-terminus (Acidic). Exons 3b and 4a are alternatively spliced coding exons yielding amino acid insertions, whereas alternatively spliced exons 2a, 2b, and 3a are predicted to be noncoding. Exons s1–s3 and 1 represent alternative 5′ UTR regions that have not been found in the same human transcript; the position of the 5′ end of exon 1 is based on currently available EST data. For more information on splicing and isoforms, see Lai et al. (2001) and Bruce and Margolis (2002). All known exons were screened for mutations, with the exception of two noncoding exons: s1 (5′ CpG-rich UTR) and 2a (alternatively spliced and untranslated). Coding variants are shown above the locus, with resulting codon and amino acid changes indicated. For reference, the KE mutation is also included. Noncoding variants are shown below the locus, with information regarding relative position (with respect to the exon in question) and frequency in probands (number of heterozygous probands/total probands screened). An asterisk (*) denotes intronic variants that correspond to those previously detected by Newbury et al. (2002), and rs numbers are indicated for polymorphisms also present in dbSNP. Locus schematic is adapted from Fisher et al. (2003).
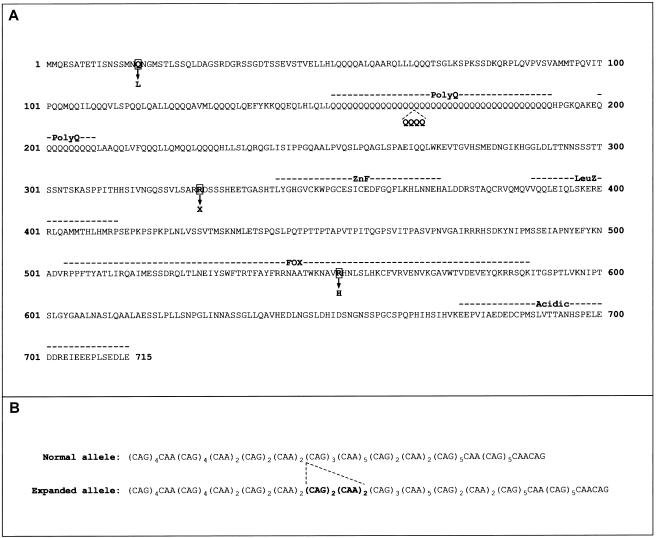
Our screening of probands also identified three novel exonic allelic variants in the coding region, each of which is predicted to yield a change in FOXP2 protein sequence (fig. 1). Crucially, one of these coding changes was a heterozygous C→T transition in exon 7, yielding a stop codon at position 328 of the FOXP2 protein (R328X) (fig. 2). We directly sequenced exon 7 in 252 control chromosomes from Human Random Control panels (obtained from the European Collection of Cell Cultures [ECACC]) but did not detect any sequence alterations, indicating that the change observed in the proband is unlikely to represent a polymorphism, according to criteria suggested by Collins and Schwartz (2002). Moreover, this nonsense mutation was also found in the affected sibling of the proband, as well as their mother, who was known to have a history of speech problems, and it was absent in their father, who has normal speech. The R328X mutation is highly likely to have functional significance, since it leads to dramatic truncation of the predicted product, yielding a FOXP2 protein lacking critical functional domains—including the zinc-finger and leucine zipper, which mediate dimerization (Wang et al. 2003), and the characteristic forkhead DNA-binding domain, known to be critical for transcription factor function (fig. 2). It is also possible that this mutation could lead to nonsense-mediated mRNA decay of transcripts from the mutant allele.
Figure 2.
A, Amino acid sequence of main isoform of human FOXP2, showing all coding changes found thus far in individuals with developmental verbal dyspraxia (Lai et al. 2001; present study). Each change was absent from large numbers of control chromosomes, but only the R328X and R553H mutations are found in multiple affected members in families segregating developmental speech and language disorder. B, Nucleotide sequence of normal and expanded polyglutamine-encoding region identified in one of the probands in this study.
The two remaining coding changes that we identified were a heterozygous A→T transversion in exon 2 in one proband, and a heterozygous insertion of the sequence CAGCAGCAACAA into the polyglutamine-encoding region of exon 5 in another. At the amino acid level, these changes are predicted to yield a glutamine-to-leucine change at residue 17 (Q17L) and a polyglutamine-tract expansion from 40 to 44 consecutive glutamines, respectively (fig. 2). Neither change was detected in screening of large numbers of chromosomes from Human Random Control panels (366 chromosomes screened for the Q17L change and 228 chromosomes screened for the polyglutamine expansion). However, in each case, the proband had a sibling who was diagnosed as affected but did not carry the relevant coding change. In addition, on the basis of current data and analyses of protein function using in silico methods (Ng and Henikoff 2001; Ramensky et al. 2002), it is not clear what the potential functional consequences of a Q17L change in FOXP2 might be. The substitution site lies in a region of unknown function close to the N-terminus of the protein but is conserved in the FOXP2 orthologues of all species thus far studied, including birds, rodents, and primates (Enard et al. 2002; Zhang et al. 2002; Webb and Zhang 2005), as well as in the closely related proteins FOXP1 and FOXP4 (Teufel et al. 2003). With regard to the polyglutamine change, although Wassink et al. (2002) previously detected small internal deletions in the Q40 tract in two families with autism, our study represents the first report of an expansion of this region in any individual. Newbury et al. (2002) previously identified a potential insertion of two CAGs within the smaller (Q10 ) tract in a family with SLI, but this did not cosegregate with the disorder, and its location in a repetitive region at the intron/exon border of exon 6 suggested that it would not have any impact on FOXP2 protein sequence. The expansion from 40 to 44 residues of the longer FOXP2 polyglutamine tract in our proband with verbal dyspraxia is outside the normal intraspecies range observed for FOXP2 orthologues; the number of consecutive glutamines in the corresponding region in other species varies from 38 glutamines (in zebra finch, chicken, and gorilla) to a maximum of 41 glutamines (in chimpanzee) (Enard et al. 2002; Zhang et al. 2002; Webb and Zhang 2005).
Our discovery of an R328X variant cosegregating with speech deficits in a multiplex family gives an opportunity to compare the neurodevelopmental consequences of a FOXP2 nonsense mutation to those of the missense mutation in the KE family. Recent clinical evaluation of cases carrying the R328X mutation suggests a similar phenotype to that associated with the R553H mutation of the KE family, where prominent difficulties in speech articulation are accompanied by more general problems with expressive and receptive language. The development of the children carrying the R328X mutation was assessed using the Griffiths (1970) Mental Development Scales, which evaluate overall development from birth to 8 years of age in six domains (“Locomotor,” “Personal and Social,” “Hearing and Speech,” “Eye/Hand Coordination,” “Performance,” and “Practical Reasoning”), while the Preschool Language Scale-3 (Zimmerman et al. 1992) was used to formally measure receptive and expressive language skills. Assessment of the proband when he was 4 years old indicated developmental delays in the domains of speech and language, and social skills. He communicated mainly using single words and was unable to repeat multisyllabic words. Eye-hand coordination was satisfactory, but he had difficulty with activities in the Practical Reasoning domain. His receptive and expressive language scores were 55 and 54, respectively (mean standard score is 100, with an SD of 15; scores between 85 and 115 are considered within normal limits). Thus, his language skills were almost 3 standard deviations below the normative mean, equivalent to that of a child of 2 years 6 mo (∼18 mo below chronological age). During informal assessment of articulation, he had difficulty in producing consonants at the beginning of words and became frustrated and significantly less intelligible during word repetition.
His younger sister has a history of motor and oropharyngeal dyspraxia, otitis media, and oesophageal reflux. On assessment using the Griffith Scales, at age 1 year 8 mo, she showed her poorest performance in the Hearing and Speech domain. She did not speak any words and could not identify objects, and her vocalization was poor. However, she was interested in puzzle-type toys and was able to put different shapes into form boards; her general motor skills at this age appeared normal. When she was subsequently assessed on the Preschool Language Scale-3 , at the age of 2 years and 11 mo, her receptive and expressive language scores were 71 and 73, respectively. Both scores were almost 2 standard deviations below the normative mean and were equivalent to the performance of an 11-mo-old child (i.e., 2 years below chronological age).
The mother, who also carried the R328X mutation, reported a history of speech delay in childhood. At present, she has severe problems with communication. She volunteered to bring a relative to the consultations, because she could not understand the nuances of what was said and was afraid of misinterpretation. She had poor speech clarity and very simple grammatical constructions. Her speech had less varied cadence than most people's, but her vocabulary was satisfactory. Her receptive difficulties were compounded by performance anxiety. Formal speech and language assessment has been offered to her and will be reported separately.
Overall, the data from our screening of cases with verbal dyspraxia support a number of important conclusions. First, the evidence indicates that one of the coding changes detected in our patients (R328X) is of direct etiological significance, since it was not found in a large panel of control chromosomes, it cosegregates with the disorder in all three affected members of a multiplex family, and it is a nonsense mutation that yields a substantially truncated protein lacking key functional domains. Thus, this is the first report of an independent causative point mutation in FOXP2 following the identification of the KE mutation by Lai et al. (2001), and the only known case of a nonsense mutation in this gene. The other coding changes (Q17L and Q40→Q44), although similarly undetected in control chromosomes, were only found in probands, and so functional studies will be important to assess whether they have any impact on the behavior of FOXP2. Second, screening of FOXP2 in <50 probands diagnosed on the basis of developmental verbal dyspraxia yielded a much higher frequency of protein sequence changes (∼6.1%) than that previously found by studying the entire coding region in children with typical SLI (no unambiguous coding mutations in 43 probands) or with autism (<0.8%; two heterozygous poly-Q deletions in a total of 239 families) (Newbury et al. 2002; Wassink et al. 2002; Gauthier et al. 2003). The frequency of unequivocal etiological variants in our sample was ∼2%. It is worth reiterating that we did not make familial clustering of the disorder a requirement when ascertaining our sample. These data suggest that a re-evaluation of the relevance of FOXP2 for common cases of neurodevelopmental disorder is warranted, and that additional coding changes might be identified in individuals with an appropriate phenotypic profile. The situation could be comparable to that found for telomere abnormalities in idiopathic mental retardation, where the former account for a small (5.1%) but significant proportion of the many individuals affected with the disorder (Flint and Knight 2003). Third, our data caution against drawing conclusions about the influence of FOXP2 from limited mutation searches that screen only small regions of the FOXP2 coding region, such as that encoding the forkhead domain (see, e.g., O’Brien et al. 2003)—especially given that nonsense mutations, like the one identified here, might lie far from known functional domains but still have major consequences for protein function. Fourth, the observation of a heterozygous nonsense mutation that severely truncates FOXP2 in multiple cases with developmental verbal dyspraxia, gives independent support to the hypothesis that FOXP2-related disorder may result from a mechanism of reduced functional gene dosage in the developing CNS.
In conclusion, the present study indicates that, whereas the neurodevelopmental consequences of FOXP2 disruption are complex, involving impairment of both speech and language skills, the presence of verbal dyspraxia is a key clinical aspect that identifies children most suitable for FOXP2 mutation screening. The identification of an unambiguous causative mutation in a cohort of <50 probands (without prior selection for familial transmission) suggests that other etiological variants may be discovered in the future. Finally, the development of individuals carrying the R328X nonsense mutation can now be assessed using neuropsychological tests and neuroimaging paradigms identical to those employed for the intensive studies of the KE family conducted over the past 15 years (see, e.g., Watkins et al. 2002; Belton et al. 2003; Liégeois et al. 2003). Therefore, our work opens the door for the future integration of behavioral and neurological data from patients with different allelic variants of FOXP2, an endeavor that will be essential for understanding the role of this gene in human cognition.
Acknowledgments
We are very grateful to all the patients and their families who took part in this study and to the clinicians who referred them. Thanks to Toril Fagerheim for help with sequencing and to W. Wetherall for his support. This work was funded by the Royal Society and the Wellcome Trust. S.C.V. is supported by the Clarendon Fund and a Christopher Welch Scholarship. A.P.M. is a Wellcome Trust Principal Research Fellow. S.E.F. is a Royal Society Research Fellow.
Appendix A
Table A1.
Primers for Mutation Screening of FOXP2 Exons
|
Primer Sequence |
||||
| Exon | Forward | Reverse | Size(bp) | Orientationa |
| s2 | 5′-CAGAAAATAATAGGAAGCTTCAACC-3′ | 5′-AAACCTGAATAGCAATAAAAACTGAG-3′ | 300 | Forward |
| s3 | 5′-TGAAGTCACTGCACTAATTGTGAT-3′ | 5′-AAGCACAATGACCAAAGGAG-3′ | 213 | Forward |
| 1 | 5′-GTAAAGCAATTGCCAAATCTACC-3′ | 5′-GATCGGGCAGAGGTGTACTCAC-3′ | 303 | Reverse |
| 2 | 5′-TAGCTTAACACAACATGCTCAG-3′ | 5′-GAGAGGGACATCTTGATAATG-3′ | 351 | Reverse |
| 2b | 5′-GGTGGTTGGCTTTGTTTCAT-3′ | 5′-GTTGGTGAGAGTTATGAGGCTAT-3′ | 495 | Forward |
| 3 | 5′-TGGGTCTGCACATCTGTTATC-3′ | 5′-GAAAGGAATATGGGAGTTCTTG-3′ | 275 | Reverse |
| 3a | 5′-ACAATGAAGGAAGTGATTACAATGC-3′ | 5′-GCACATACACACATGCACACTTCC-3′ | 275 | Reverse |
| 3b | 5′-CATATAATCTATTTAGTGGGACTGG-3′ | 5′-CTTCCCTATTTTGACCTACCAAG-3′ | 281 | Reverse |
| 4 | 5′-GATAACATACTATTTGTGAAGTTG-3′ | 5′-TCTAGCACGCTAATAGGTTGTCC-3′ | 346 | Forward |
| 4a | 5′-CAACTGGTAGAGTATAGCCTAGTTTTT-3′ | 5′-TGTTGTTGCTGCTGCTGT-3′ | 450 | Forward |
| 5 | 5′-TGAATCTTAATGGATACTCTGCC-3′ | 5′-TCTAAGACTATTCTTGCCGCTC-3′ | 385 | Forward |
| 6 | 5′-AAGGAGTGTGCATTTCCCTG-3′ | 5′-CAGAAAGGCCATGAAATGGTAG-3′ | 330 | Forward |
| 7 | 5′-AATTTATGCAGGTAACATCACTG-3′ | 5′-CTTTTTCATTGTCTCAATGGTG-3′ | 405 | Forward |
| 8 | 5′-GTGCCTAAAATGCCCATATAATCC-3′ | 5′-TGTTTGTCACTGATCGTAACCTG-3′ | 260 | Reverse |
| 9 | 5′-GCTTTTTAAGTGTAGCCTATGCC-3′ | 5′-CAGAATCTGCTCAGTACTCAATG-3′ | 235 | Forward |
| 10 | 5′-GAGGCAAGCTCAATGATAAGATG-3′ | 5′-CACCCATGGTGTAGATTGGATAG-3′ | 384 | Forward |
| 11 | 5′-TTGATTCAGCTACAGTTTTCCTC-3′ | 5′-AATTAGTTGAGATTGGCTGCTTC-3′ | 410 | Reverse |
| 12 | 5′-ATGACACTTTCTCATCCAAGCC-3′ | 5′-CTTCCTCACTGAATCACTTTACC-3′ | 264 | Reverse |
| 13 | 5′-CCTTGTGCTATCTGTAAGGAAC-3′ | 5′-AGTAAGGCTTGGATGAGAAAGTG-3′ | 245 | Reverse |
| 14 | 5′-CATTGCCACGAGAATGTTAGC-3′ | 5′-TCAATAATGTAGTATGTTGGGCTG-3′ | 323 | Reverse |
| 15 | 5′-GTGTGAGACAAGCCAGAACATAC-3′ | 5′-CTTGAATTAGAACCAGTGCTGTC-3′ | 331 | Forward |
| 16 | 5′-CTGCCACAAGTAGCCAGTTAGG-3′ | 5′-CAACTTATACAACAGTAAAAACTTCG-3′ | 424 | Forward |
| 17 | 5′-TGACCTCTTCACTGCAAAGTTGG-3′ | 5′-GTCAAATATTCATGGTTGTGGAG-3′ | 303 | Forward |
Orientation of “F” primer with respect to the transcription of the FOXP2 locus.
Electronic-Database Information
The URLs for data presented herein are as follows:
- dbSNP Home Page, http://www.ncbi.nlm.nih.gov/SNP/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
References
- American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders (DSM-IV). American Psychiatric Association, Washington, DC [Google Scholar]
- Belton E, Salmond CH, Watkins KE, Vargha-Khadem F, Gadian DG (2003) Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum Brain Mapp 18:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce HA, Margolis RL (2002) FOXP2: novel exons, splice variants, and CAG repeat length stability. Hum Genet 111:136–144 [DOI] [PubMed] [Google Scholar]
- Collins JS, Schwartz CE (2002) Detecting polymorphisms and mutations in candidate genes. Am J Hum Genet 71:1251–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enard W, Przeworski M, Fisher SE, Lai CSL, Wiebe V, Kitano T, Monaco AP, Pääbo S (2002) Molecular evolution of FOXP2, a gene involved in speech and language. Nature 418:869–872 [DOI] [PubMed] [Google Scholar]
- Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA (2003). Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol 460:266–279 [DOI] [PubMed] [Google Scholar]
- Fisher SE, Lai CSL, Monaco AP (2003) Deciphering the genetic basis of speech and language disorders. Annu Rev Neurosci 26:57–80 [DOI] [PubMed] [Google Scholar]
- Flint J, Knight S (2003) The use of telomere probes to investigate submicroscopic rearrangements associated with mental retardation. Curr Opin Genet Dev 13:310–316 [DOI] [PubMed] [Google Scholar]
- Gauthier J, Joober R, Mottron L, Laurent S, Fuchs M, De Kimpe V, Rouleau GA (2003) Mutation screening of FOXP2 in individuals diagnosed with autistic disorder Am J Med Genet 118A:172–175 [DOI] [PubMed] [Google Scholar]
- Griffiths R (1970) The abilities of young children. Child Development Research Centre, London [Google Scholar]
- Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C (2004) FoxP2 expression in avian vocal learners and non-learners. J Neurosci 24:3164–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminen N, Hannula-Jouppi K, Kestila M, Lahermo P, Muller K, Kaaranen M, Myllyluoma B, Voutilainen A, Lyytinen H, Nopola-Hemmi J, Kere J (2003) A genome scan for developmental dyslexia confirms linkage to chromosome 2p11 and suggests a new locus on 7q32. J Med Genet 40:340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP (2001) A novel forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413:519–523 [DOI] [PubMed] [Google Scholar]
- Lai CSL, Gerrelli D, Monaco AP, Fisher SE, Copp AJ (2003) FOXP2 expression during brain development coincides with sites of pathology in a severe speech and language disorder. Brain 126:2455–2462 [DOI] [PubMed] [Google Scholar]
- Lewis BA, Freebairn LA, Hansen A, Gerry Taylor H, Iyengar S, Shriberg LD (2004) Family pedigrees of children with suspected childhood apraxia of speech. J Commun Disord 37:157–175. [DOI] [PubMed] [Google Scholar]
- Liégeois F, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha-Khadem F (2003) Language fMRI abnormalities associated with FOXP2 gene mutation. Nature Neurosci 6:1230–1237 [DOI] [PubMed] [Google Scholar]
- Liégeois FJ, Lai CSL, Baldeweg T, Fisher SE, Monaco AP, Connelly A, Vargha-Khadem, F (2001) Behavioural and neuroimaging correlates of a chromosome 7q31 deletion containing the SPCH1 gene. Abstr Soc Neurosci 27:529.17 [Google Scholar]
- Marcus GF, Fisher SE (2003) FOXP2 in focus: what can genes tell us about speech and language? Trends Cogn Sci 7:257–262 [DOI] [PubMed] [Google Scholar]
- Newbury DF, Bonora E, Lamb JA, Fisher SE, Lai CSL, Baird G, Jannoun L, Slonims V, Stott CM, Merricks MJ, Bolton PF, Bailey A, Monaco AP, International Molecular Genetic Study of Autism Consortium (2002) FOXP2 is not a major susceptibility gene for autism or specific language impairment. Am J Hum Genet 70:1318–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PC, Henikoff S (2001) Predicting deleterious amino acid substitutions. Genome Res 11:863–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien EK, Zhang X, Nishimura C, Tomblin JB, Murray JC (2003) Association of specific language impairment (SLI) to the region of 7q31. Am J Hum Genet 72:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramensky V, Bork P, Sunyaev S (2002) Human non-synonymous SNPs: server and survey. Nucleic Acids Res 30:3894–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem RA, Banerjee-Basu S, Berry FB, Baxevanis AD, Walter MA (2003) Structural and functional analyses of disease-causing missense mutations in the forkhead domain of FOXC1. Hum Mol Genet 12:2993–3005 [DOI] [PubMed] [Google Scholar]
- Shu W, Yang H, Zhang L, Lu MM, Morrisey EE (2001) Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem 276:27488–27497 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Liu FC, Hirokawa K, Takahashi H (2003) Expression of Foxp2, a gene involved in speech and language, in the developing and adult striatum. J Neurosci Res 73:61–72 [DOI] [PubMed] [Google Scholar]
- Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA (2004) Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J Neurosci 24:3152–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel A, Wong EA, Mukhopadhyay M, Malik N, Westphal H (2003) FoxP4, a novel forkhead transcription factor. Biochim Biophys Acta 1627:147–152 [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, Frackowiak RS, Friston KJ, Pembrey ME, Mishkin M, Gadian DG, Passingham RE (1998) Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci USA 95:12695–12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Copp A, Mishkin M (2005) FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci 6: 131–138 [DOI] [PubMed] [Google Scholar]
- Wang B, Lin D, Li C, Tucker P (2003) Multiple domains define the expression and regulatory properties of Foxp1 forkhead transcriptional repressors. J Biol Chem 278:24259–24268 [DOI] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, Sheffield VC (2002) Evaluation of FOXP2 as an autism susceptibility gene. Am J Med Genet 114:566–569 [DOI] [PubMed] [Google Scholar]
- Watkins KE, Dronkers NF, Vargha-Khadem F (2002) Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain 125:452–464 [DOI] [PubMed] [Google Scholar]
- Webb DM, Zhang J (2005) FoxP2 in song-learning birds and vocal-learning mammals. J Hered 96:212–216 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1993) The ICD-10 classification for mental and behavioural disorders: diagnostic criteria for research. World Health Organisation, Geneva [Google Scholar]
- Zhang J, Webb DM, Podlaha O (2002) Accelerated protein evolution and origins of human-specific features: Foxp2 as an example. Genetics 162:1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VC, Evatt Pond R (1992) The preschool language scale. Psychological Corporation, San Antonio [Google Scholar]