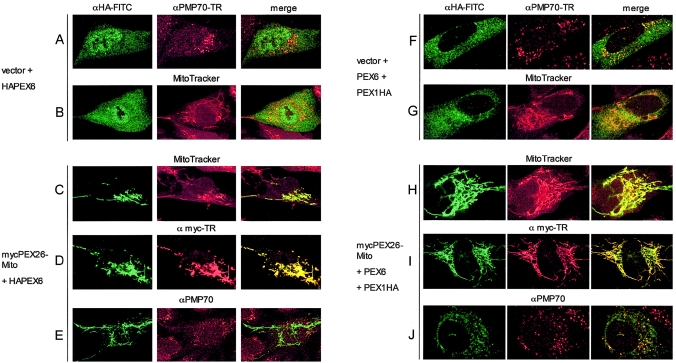
Figure 8.
Expression of functional PEX26-Mito completely mislocalizes PEX6 and partially mislocalizes PEX1 to mitochondrial membranes. We analyzed PBD059 cells (c.230+1G→T/c.230+1G→T) by indirect immunofluorescence with anti-HA-FITC and anti-myc-Texas Red, as indicated. To mark peroxisomal or mitochondrial membranes, we used anti-PMP70 (Texas Red) or Mito-Tracker labeling (red), respectively. Yellow color in the merged column indicates colocalization. A–E, PBD059 cells were transfected with pcDNA3-HAPEX6 and either nonrecombinant pcDNA3 vector or recombinant pcDNA3 expressing mycPEX26-Mito (mycPEX26 [aa 2–222])–OMP25 [aa 170–206]). Panels A and B show a lack of colocalization of HAPEX6 with peroxisomes (A) or mitochondria (B) in cells cotransfected with pcDNA3 vector plus a recombinant vector expressing HAPEX6. By contrast, coexpression of mycPEX26-Mito redirects HAPEX6 to mitochondrial membranes (C), where it colocalizes with PEX26-Mito (D) but not with peroxisomes (E). In panels F–J, PBD059 cells were transfected with pcDNA3-PEX6 and pcDNA3-PEX1HA plus either nonrecombinant pcDNA3 (F and G) or recombinant pcDNA3 expressing mycPEX26-Mito (H–J). Panels F and G show a bimodal distribution of PEX1HA in the cytoplasm and on peroxisomes but no colocalization with mitochondria (G). Coexpression of mycPEX26-Mito redirects PEX1HA to mitochondrial membranes to a varying degree (H–J), with a fraction of PEX1 retaining a residual peroxisomal location (J). In multiple experiments, the amount of PEX1 on peroxisomes varied; in panel J, we show a typical ratio of peroxisomal to mitochondrial location of PEX1 from observations in three independent cotransfection experiments.