Abstract
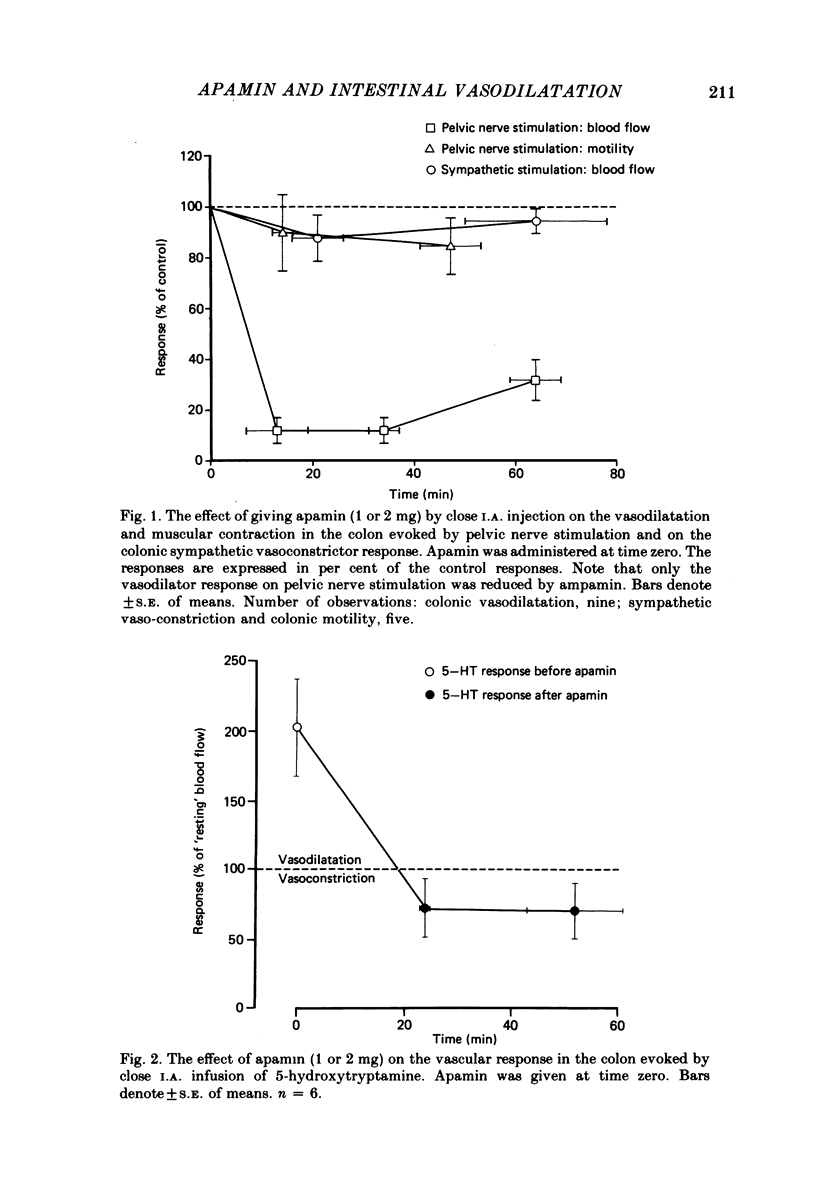
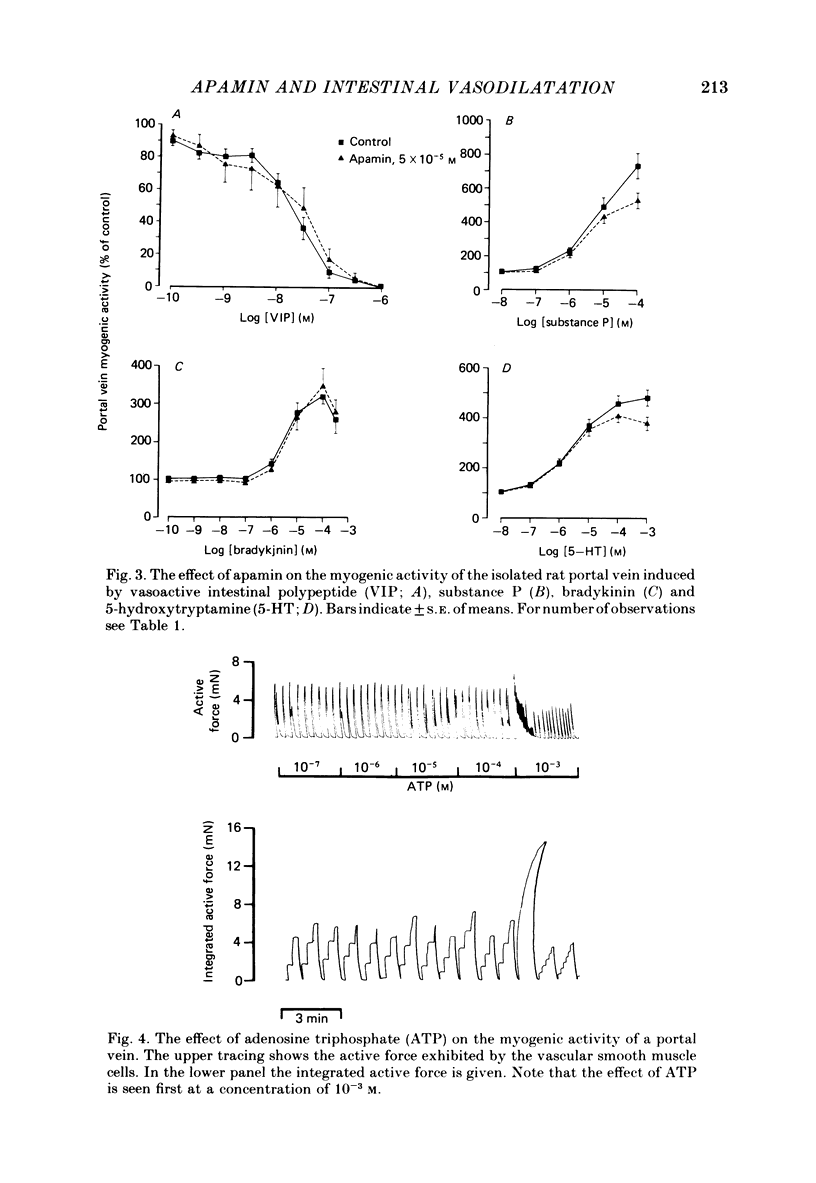
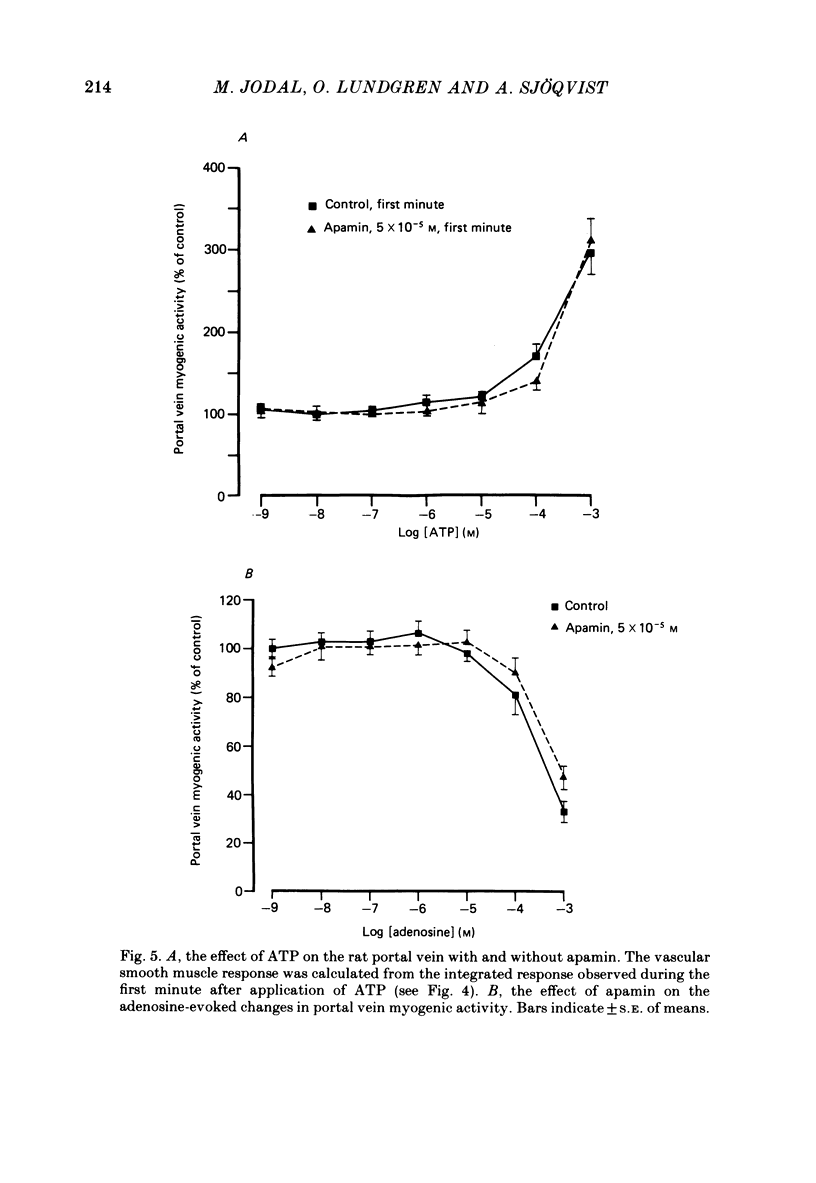
The effects of apamin, a polypeptide isolated from bee venom, on different vasodilator mechanisms in the small and large intestines were studied in atropinized cats. In the large intestine vasodilatation in response to pelvic nerve stimulation was either abolished or markedly diminished by I.A. apamin. However, neither the contraction of colonic muscle which occurred under these conditions nor sympathetic vasoconstriction was significantly influenced by apamin, suggesting that the effect of the peptide was not a non-specific effect on nerves or vascular smooth muscle. In the small intestine it was observed that the nervous vasodilatation induced by transmural electrical field stimulation or mechanical mucosal stimulation was either diminished or abolished by apamin. Intestinal vasodilatation, caused by close I.A. infusions of 5-hydroxytryptamine (5-HT), was abolished by apamin. After giving apamin 5-HT infusions induced a vasoconstriction in five out of six experiments. Vasodilatation induced by vasoactive intestinal polypeptide (VIP) was not significantly affected by apamin. In a series of in vitro experiments on rat portal vein, dose-response curves of several putative intestinal neurotransmitters were determined in the presence and absence of apamin. The following substances were tested: VIP, substance P, bradykinin, 5-HT, ATP and adenosine. Apamin had no effect on the dose-response curves of any of these compounds. The results are discussed in relation to the possibility that apamin may act by blocking the release of a putative peptidergic transmitter from nerve terminals.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson H. Studies on the inhibitory nervous control of gastric motility. Acta Physiol Scand Suppl. 1973;390:1–38. [PubMed] [Google Scholar]
- Andersson P. O., Järhult J. Separation of colonic motor and blood flow responses to pelvic nerve stimulation in the cat. Acta Physiol Scand. 1981 Oct;113(2):263–265. doi: 10.1111/j.1748-1716.1981.tb06893.x. [DOI] [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber B., Fara J., Lundgren O. Intestinal vascular responses to 5-hydroxytryptamine. Acta Physiol Scand. 1973 Apr;87(4):526–534. doi: 10.1111/j.1748-1716.1973.tb05419.x. [DOI] [PubMed] [Google Scholar]
- Biber B., Fara J., Lundgren O. Intestinal vasodilatation in response to transmural electrical field stimulation. Acta Physiol Scand. 1973 Feb;87(2):277–282. doi: 10.1111/j.1748-1716.1973.tb05391.x. [DOI] [PubMed] [Google Scholar]
- Biber B., Jodal M., Lundgren O., Svanvik J. Intestinal vasodilatation after mechanical stimulation of the jejunal mucosa. Experientia. 1970 Mar 15;26(3):263–264. doi: 10.1007/BF01900084. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Burnstock G. Evidence in support of the P1/P2 purinoceptor hypothesis in the guinea-pig taenia coli. Br J Pharmacol. 1981 Jul;73(3):617–624. doi: 10.1111/j.1476-5381.1981.tb16796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Eklund S., Fahrenkrug J., Jodal M., Lundgren O., Schaffalitzky de Muckadell O. B., Sjöqvist A. Vasoactive intestinal polypeptide, 5-hydroxytryptamine and reflex hyperaemia in the small intestine of the cat. J Physiol. 1980 May;302:549–557. doi: 10.1113/jphysiol.1980.sp013260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Haglund U., Jodal M., Lundgren O., Olbe L., de Muckadell O. B. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. J Physiol. 1978 Nov;284:291–305. doi: 10.1113/jphysiol.1978.sp012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasth S., Hultén L., Nordgren S., Zeitlin I. J. Studies on the atropine-resistant sacral parasympathetic vascular and motility responses in the cat colon. J Physiol. 1981 Feb;311:421–429. doi: 10.1113/jphysiol.1981.sp013594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5(1):1–20. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B. The effects of botulinum toxin on the synthesis, storage and release of acetylcholine. Prog Neurobiol. 1980;14(2-3):99–119. doi: 10.1016/0301-0082(80)90019-2. [DOI] [PubMed] [Google Scholar]
- HILTON S. M., LEWIS G. P. The cause of the vasodilatation accompanying activity in the submandibular salivary gland. J Physiol. 1955 May 27;128(2):235–248. doi: 10.1113/jphysiol.1955.sp005302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILTON S. M., LEWIS G. P. The mechanism of the functional hyperaemia in the submandibular salivary gland. J Physiol. 1955 Aug 29;129(2):253–271. doi: 10.1113/jphysiol.1955.sp005351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Haglund U., Lundgren O. Reactions within consecutive vascular sections of the small intestine of the cat during prolonged hypotension. Acta Physiol Scand. 1972 Feb;84(2):151–163. doi: 10.1111/j.1748-1716.1972.tb05166.x. [DOI] [PubMed] [Google Scholar]
- Haux P., Sawerthal H., Habermann E. Sequenzanalyse des Bienengift-Neurotoxins (Apamin) aus seinen tryptischen und chymotryptischen Spaltstücken. Hoppe Seylers Z Physiol Chem. 1967 Jun;348(6):737–738. [PubMed] [Google Scholar]
- Hirst G. D. Mechanisms of peristalsis. Br Med Bull. 1979 Sep;35(3):263–268. doi: 10.1093/oxfordjournals.bmb.a071587. [DOI] [PubMed] [Google Scholar]
- Ljung B. Nervous and myogenic mechanisms in the control of a vascular neuroeffector system. Acta Physiol Scand Suppl. 1970;349:33–68. [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A., Ras R., Van den Akker J. The action of apamin on guinea-pig taenia caeci. Eur J Pharmacol. 1980 Oct 17;67(2-3):265–274. doi: 10.1016/0014-2999(80)90507-5. [DOI] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A. The effect of apamin on the smooth muscle cells of the guinea-pig taenia coli. Eur J Pharmacol. 1979 Sep 15;58(2):151–156. doi: 10.1016/0014-2999(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Maas A. J. The effects of apamin on responses evoked by field stimulation in guinea-pig taenia caeci. Eur J Pharmacol. 1981 Jul 17;73(1):1–9. doi: 10.1016/0014-2999(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Mackenzie I., Burnstock G. Evidence against vasoactive intestinal polypeptide being the non-adrenergic, non-cholinergic inhibitory transmitter released from nerves supplying the smooth muscle of the guinea-pig taenia coli. Eur J Pharmacol. 1980 Oct 17;67(2-3):255–264. doi: 10.1016/0014-2999(80)90506-3. [DOI] [PubMed] [Google Scholar]
- Muller M. J., Baer H. P. Apamin, a nonspecific antagonist of smooth muscle relaxants. Naunyn Schmiedebergs Arch Pharmacol. 1980 Feb;311(1):105–107. doi: 10.1007/BF00500310. [DOI] [PubMed] [Google Scholar]
- Ottesen B. Vasoactive intestinal polypeptide (VIP): effect on rabbit uterine smooth muscle in vivo and in vitro. Acta Physiol Scand. 1981 Oct;113(2):193–199. doi: 10.1111/j.1748-1716.1981.tb06882.x. [DOI] [PubMed] [Google Scholar]
- Shuba M. F., Vladimirova I. A. Effect of apamin on the electrical responses of smooth muscle to adenosine 5'-triphosphate and to non-adrenergic, non-cholinergic nerve stimulation. Neuroscience. 1980;5(5):853–859. doi: 10.1016/0306-4522(80)90154-2. [DOI] [PubMed] [Google Scholar]
- Sjöberg B., WAHLSTRöm B. A. The effect of ATP and related compounds on spontaneous mechanical activity in the rat portal vein. Acta Physiol Scand. 1975 May;94(1):46–53. doi: 10.1111/j.1748-1716.1975.tb05860.x. [DOI] [PubMed] [Google Scholar]
- Sjöqvist A., Delbro D., Jodal M., Lundgren O. The effect of Apamin on nonadrenergic, noncholinergic nervous vasodilatations in the cat small intestine. Experientia. 1980 Oct 15;36(10):1202–1202. doi: 10.1007/BF01976128. [DOI] [PubMed] [Google Scholar]


