Abstract
Asthma, like many inflammatory disorders, is affected by psychological stress, suggesting that reciprocal modulation may occur between peripheral factors regulating inflammation and central neural circuitry underlying emotion and stress reactivity. Despite suggestions that emotional factors may modulate processes of inflammation in asthma and, conversely, that peripheral inflammatory signals influence the brain, the neural circuitry involved remains elusive. Here we show, using functional magnetic resonance imaging, that activity in the anterior cingulate cortex and insula to asthma-relevant emotional, compared with valence-neutral stimuli, is associated with markers of inflammation and airway obstruction in asthmatic subjects exposed to antigen. This activation accounts for ≥40% of the variance in the peripheral markers and suggests a neural basis for emotion-induced modulation of airway disease in asthma. The anterior cingulate cortex and insula have been implicated in the affective evaluation of sensory stimulation, regulation of homeostatic responses, and visceral perception. In individuals with asthma and other stress-related conditions, these brain regions may be hyperresponsive to disease-specific emotional and afferent physiological signals, which may contribute to the dysregulation of peripheral processes, such as inflammation.
Keywords: brain–periphery interaction, functional magnetic resonance imaging, inflammation, anterior cingulate cortex, insula
Chronic diseases that are characterized by dysregulation of inflammation, such as asthma, are particularly susceptible to modulation by stress and emotion (1, 2), suggesting that inflammation is a likely final common pathway linking neural circuitry underlying emotion with symptom aggravation. In an investigation of these linkages, Liu and colleagues (2) have shown that undergraduate asthmatic subjects had greater airway inflammation and decreased lung function to an allergen inhalation challenge during final examination week, a period of significantly heightened stress, compared with an identical challenge during a relatively stress-free period. Others have shown that immune cell cytokine profiles shift toward the promotion of an allergic response and inflammation during prolonged stress (3, 4). Despite the compelling support for a model integrating psychological and physiological factors in asthma, the brain has been largely absent from any discussion of its mechanistic underpinnings. The extant literature indicates that both physiological and psychological stressors activate similar neural circuitry, acting as two different routes to a bidirectional communication network between the brain and the immune system (5, 6). Consistent with this model, neural circuitry underlying stress and emotion can regulate inflammation (7, 8), and peripheral inflammatory mediators can influence mood and cognitive function (9). Depressive symptomatology, for instance, has been associated with elevations in the same proinflammatory cytokines that are released during an asthmatic reaction (10, 11). Thus, we sought to identify a specific neural circuitry through which cognitive and emotional factors may interact with the physiological events of an acute asthmatic response to influence the severity of asthma symptom expression.
Our hypotheses concerning the constituents of this neural circuitry focused on the insula and anterior cingulate cortex (ACC). Both receive afferent input from the lamina I spinothalamocortical tract, carrying information pertaining to the physiological condition of the body (12) (e.g., shortness of breath; refs. 13 and 14), and have strong connections with neural structures essential in processing emotional information (15, 16). Results of lesion studies support this view, because focal damage to the anterior insula results in a decrease in the affective component of pain and disrupts its associated homeostatic consequences (17). In addition, neuroimaging data reveal that the magnitude of insular activation predicts individual differences in evaluation of stimulus intensity (18) and visceral awareness (19). Likewise, the ACC has been implicated in the affective and motivational aspects of sensation, as illustrated by an increase in activation during hypnotic suggestion of the increasing unpleasantness of a constant stimulus (20). Lesions to this area result in a reduction in motivation to avoid a painful stimulus (without disruption of pain perception) (21) and in the failure of contextually appropriate sympathetic activity (22).
In asthma, the inhalation of allergen causes acute pulmonary mast cell degranulation and mediator release to initiate bronchial smooth muscle contraction that is characteristic of the early phase of an allergic reaction. Other cells, including lymphocyte subpopulations (e.g., Th2), are activated and release cytokines that initiate an inflammatory response 4–8 h later. This inflammatory response leads to a late-phase reaction involving the recruitment of eosinophils (EOS) and reappearance of airway obstruction. Unlike that of the early phase, late-phase airway obstruction is attributed to inflammation, making it of primary interest when examining dysregulation of inflammatory processes as a mechanism underlying the association of stress and emotion with symptom expression in asthma.
The ability of glucocorticoids (GC) to constrain an inflammatory response is critical in preventing excessive tissue damage. The important role of endogenous GCs, in this regard, is illustrated by the increase in susceptibility to inflammatory disease in rats bred for a reduced ability to produce GCs (23). The ability of GCs to inhibit inflammation is determined not only by the amount produced, but also by their interaction with immune cells. Psychological stress can result in a decreased sensitivity of immune cells to inhibition by GCs (24, 25), which may partially underlie the stress-induced symptom exacerbation in inflammatory diseases. Likewise, increased production of proinflammatory cytokines, due to diminished GC inhibition, can impact psychological function. Although it is unclear what induces the reduction in sensitivity, it is possible that output from affective neural circuitry, through the sympathetic nervous system, may down-regulate GC receptors on immune cells (26).
The present study used the late-phase component of an allergic asthmatic reaction to inhaled antigen as a model to identify brain regions activated by emotional and physiological cues, which may participate in the development and regulation of inflammation in the lungs. Functional magnetic resonance imaging was used to noninvasively image brain function in response to asthma-relevant emotional stimuli after an inhalation challenge. We predicted that the insula and ACC would show differences in activation in response to asthma-relevant (As) stimuli, compared with negative (Ng) or valence-neutral (Ne) stimuli, during an antigen (Ag) relative to a methacholine (Meth) or saline challenge. Further, we anticipated this differential activation only during the late-phase component of the allergic response because we hypothesized that this activation reflects modulation in response to an inflammatory signal associated with the late-phase allergic response. In addition, we predicted that individual differences in this differential activation would be associated with the magnitude of the peripheral physiological response to Ag.
Methods
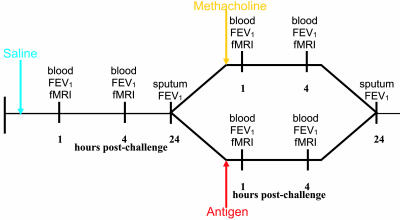
Experimental Design. Asthmatic participants each underwent three inhalation challenges (Fig. 1), separated by a minimum of 4 wk: saline (baseline control); Meth, an acute bronchoconstrictor without the capacity to cause inflammation; and subject-specific Ag that caused an early and late-phase response. Administration of Meth and Ag challenge was double-blind, and the assignment was counterbalanced to the second and third scanning session. This design was used as a means of parsing changes in brain activation associated with Ag exposure and subsequent inflammation from those associated with bronchoconstriction per se and enabled us to identify potential CNS activity associated with the inflammatory component of an asthma attack.
Fig. 1.
Experimental design: timing of experimental challenges and measures collected. Participants underwent both Ag and Meth challenges separated by at least 4 wk in a complete within-subjects crossover design. Challenge order was counterbalanced.
For each challenge, functional magnetic resonance imaging scans were performed at 1 h and 4 h after the challenge, timed to coincide with the resolution of the early phase and the onset of the late phase, respectively. There were thus six separate functional magnetic resonance imaging sessions conducted per subject. During neuroimaging, participants performed a reaction time task where they identified the color of As, negative, or Ne words. Lung function (forced expiratory volume in 1 s, FEV1) and local inflammatory potential (sputum EOS) were measured before, during, and after the challenge. GC inhibition of peripheral blood leukocyte (PBL) cytokine production was used to assess the ability of GCs to modulate inflammation and was measured at 1 and 4 h after the challenge.
Participants. Six participants (three female) with mild allergic asthma were recruited for the study. Each participant had a positive skin-prick test to cat dander, house dust mite, or ragweed extract and had a history of asthma with previous use of asthma medications. All participants demonstrated both an early phase response (>20% decrease in FEV1 within 1 h of the Ag challenge) and a late-phase response (>15% decrease in FEV1 4–8 h after the Ag challenge) to inhaled allergen challenge during screening. During the study, no participants required inhaled corticosteroids, had evidence of a respiratory infection, or had an exacerbation of their asthma within the previous 4 wk.
Lung Function and Inflammation Measurement. Lung function was measured according to American Thoracic Society standards (27) every 15 min until 45 min after early response by using FEV1. In addition, lung function was measured hourly for 8 h by using portable spirometry equipment. Response to a challenge (saline, Meth, and Ag) was measured as a change in FEV1 when compared with the prechallenge values.
Inflammatory cell differentials were measured in induced sputum. Sputum inductions were performed as described in ref. 28 before and 24 h after the challenge. Differentials (300 cells per slide) were reported as the percentage of monocytes/macrophages, lymphocytes, neutrophils, and eosinophils.
Inhalation Challenges. Allergen challenges were performed by using subject-specific, commercially available allergen extract (house dust mite extract, n = 3; ragweed extract, n = 3). Briefly, subjects inhaled nebulized escalating doses of allergen extract (Ag challenge) and Provocholine (Methapharm, Brantford, ON, Canada) (Methacholine challenge). Spirometry was performed after each dose of challenge material, and the challenge was stopped when a subject's baseline FEV1 decreased by at least 20% compared with the challenge baseline.
Glucocorticoid Sensitivity. Blood was collected in heparinized tubes at the baseline and 1 and 4 h after the challenge. Whole blood was incubated with lippopolysaccharide (LPS), and dexamethasone (DEX; a synthetic glucocorticoid) for 20 h at 37°C and 5% CO2 (29). LPS, a bacterial cell wall component, activates PBLs, causing them to release proinflammatory cytokines. DEX will attenuate immune activation and decrease cytokine production. Supernatants were analyzed for TNF-α by using a sandwich ELISA. DEX inhibition of cytokine production was computed as TNF-α production in the presence of DEX relative (%) to TNF-α production without DEX, with baseline values removed. The resulting value for the Meth challenge was then subtracted from that of the Ag challenge to obtain a measure reflecting decrease in GC sensitivity during Ag relative to Meth challenge. The resulting difference score was used in subsequent analyses.
Brain Image Acquisition. A GE Signa 3.0-Tesla high-speed imaging device with a quadrature head coil (General Electric Medical Systems, Milwaukee, WI) was used to acquire both anatomical and whole-brain functional images. Two runs of functional images consisted of 30 × 4-mm sagittal Echo-Planar Imaging slices covering the whole brain [1-mm interslice gap; 64 × 64 in-plane resolution, 240-mm field of view (FOV); repetition time/echo time/flip = 2,000 ms/30 ms/60]. Immediately preceding acquisition of functional images, a whole-brain high-resolution T1-weighted anatomical scan (3D T1-weighted inversion recovery fast gradient echo; 256 × 256 in-plane resolution, 240 mm FOV; 124 × 1.1 mm axial slices) was acquired. Head movement was minimized by using a vacuum pillow (S & S Par Scientific, Houston).
Imaging Task. The stimuli were 20 As (e.g., wheeze), 20 negative (e.g., loneliness), and 20 valence-neutral (e.g., curtains) words presented digitally in one of four colors (blue, yellow, green, or red) by using e-prime software (Psychology Software Tools, Pittsburgh). As words were words associated with an asthmatic episode generated by asthmatics. Negative and Ne words were selected from the ANEW data set (30). Each set was matched on word length, usage frequency, and part of speech. Stimuli were presented by using the VisuaStim XGA Goggle System (Resonance Technology, Northridge, CA) with a resolution of 800 × 600 pixels. We instructed participants to identify the color of each stimulus by pressing one of four buttons on an MRI-compatible response pad (Current Designs, Philadelphia). The task and the color-button associations were learned before the first scanning session and practiced during the collection of anatomical images immediately before collection of the first functional scan. Reaction time and accuracy were recorded by using e-prime. The start of stimulus presentation was synchronized with the start of the scanner. A 20-s baseline period of functional data collection flanked each experimental run. In each run, 30 stimuli (10 per category in random order) were presented for 2 s each with a pseudorandomized interstimulus interval of 8–12 s.
Data Preprocessing. All off-line data processing was done by using analysis of functional neuro images (afni) software (31). After image reconstruction, each time series was corrected for motion by realigning it with the first acquired image. Individual subject data for each run were then analyzed by using a general linear model (GLM) deconvolution with separate regressors for each experimental condition (i.e., As, Ng, and Ne), to estimate the hemodynamic response. The GLM yielded a set of area under the curve contrast maps (As - Ne, Ng - Ne, Ag - Ng, As - fixation, Ng - fixation, and Ne - fixation) for each individual. These contrast maps were then transformed into Talairach Space and spatially blurred with a 7-mm full-width at half-maximum Gaussian spatial filter.
Statistical Analyses. Asthma-relevant stimuli were compared with Ng and Ne stimuli to identify brain regions sensitive to contextually specific cues. The statistical significance of main contrast effects (i.e., those that remain stable over scan sessions) and session effects (i.e., changes in contrasts across scan sessions) were tested by entering contrast maps for all subjects into a mixed effects analysis with subjects as a random factor and challenge (Meth vs. Ag), and phase (early vs. late) as fixed factors. A whole-brain search was conducted to identify regions with activation showing a challenge × phase interaction in each contrast map (e.g., As - Ne).
To address the prediction that individual differences in the magnitude of response in the ACC and insula to As stimuli during Ag challenge would be associated with changes in the peripheral measures of lung function and inflammation, we tested the correlation between neural activation and each peripheral measure. For each participant, difference images were created for each valence condition by subtracting the contrast map for the Meth challenge from the corresponding contrast map for the Ag challenge [e.g., (As - Ne) for Ag - (As - Ne) for Meth]. We conducted a whole-brain search for regions showing relations between measures of lung function and between markers of inflammatory processes. Images were thresholded by using an uncorrected voxelwise threshold of P < 0.01, combined with a cluster size threshold of 248 mm3. This combined threshold was estimated with a Monte Carlo simulation by using afni (alphasim) to give a one-tailed corrected P < 0.05, based on a gray matter a priori search volume limited to the anterior parts of the brain (Talairach coordinates y > -35).
In addition to using the difference in brain activation during Ag relative to Meth challenge to predict peripheral measures, we examined the unique association of brain activity during each of the challenges (As - Ne contrast) with the changes in the peripheral measures. We entered the percent signal change extracted from the As - Ne contrast map for the Ag and Meth challenges as two independent variables in a regression analysis predicting the Ag - Meth difference score for each peripheral measure. This approach enabled us to determine the relative contribution of brain modulation during each challenge to the difference in peripheral measures between the two challenges. We predicted that changes in brain activity occurring during the Ag challenge would be primarily responsible for associations with the periphery that were identified in the correlational analyses.
Results
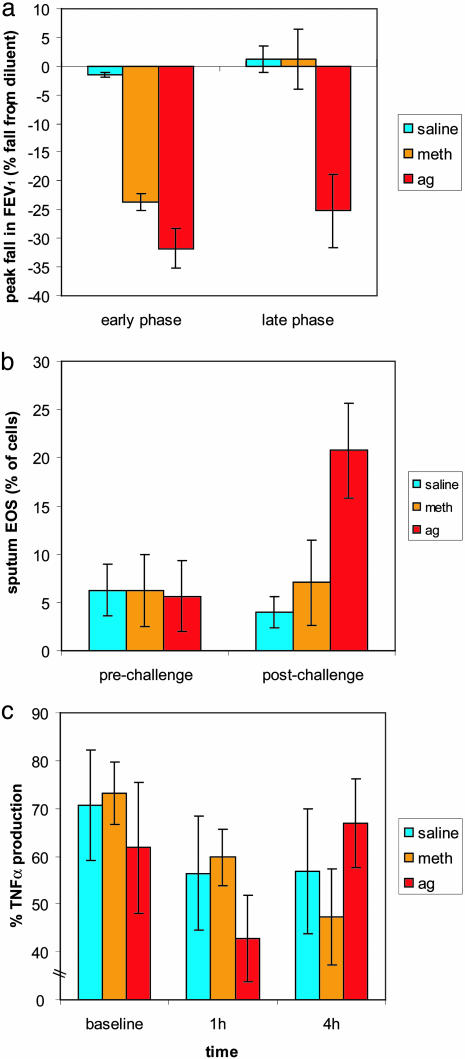
Peripheral Measures. There was no change in FEV1 at any time during the saline challenge. Both Meth and Ag caused a similar fall in FEV1 immediately after the challenge. After returning to baseline, there was a secondary phase decrease in FEV1, measured 4–8 h after the challenge, only following the Ag challenge (Fig. 2a; F (2, 4) = 24.2, P < 0.01; for timecourse, see also Fig. 5, which is published as supporting information on the PNAS web site). Sputum EOS, measured 24 h after the challenge, increased significantly only after the Ag challenge (Fig. 2b; F (2, 4) = 26.4, P < 0.01), verifying the specificity of this marker of inflammation to the Ag challenge. Compared with the control (saline) and Meth conditions, GC inhibition of TNF-α production was diminished during the Ag challenge (Fig. 2c; F (4, 16) = 5.06, P < 0.01). These observations suggest that the PBLs are responding to a physiological signal that is unique to the Ag challenge.
Fig. 2.
Greater inflammatory potential and decreased lung function during an Ag challenge. (a) Peak fall in FEV1 during the early (1 h) and late phase (6–8 h) of each challenge (phase × challenge interaction; F (2, 4) = 24.2, P < 0.01). (b) Percentage of EOS before and 24 h after the challenge (time × challenge interaction; F (2, 4) = 26.4, P < 0.01). (c) PBL production of TNF-α (% of production relative to no DEX) at baseline (before), early phase (1 h after) and late phase (4 h after) (phase × challenge interaction; F (4, 16) = 5.06, P < 0.01). Error bars represent standard error of the mean.
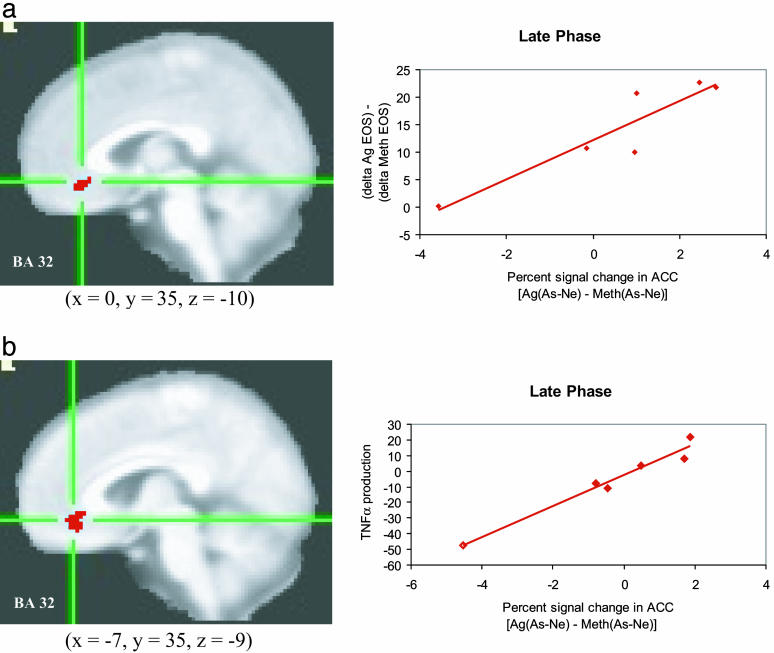
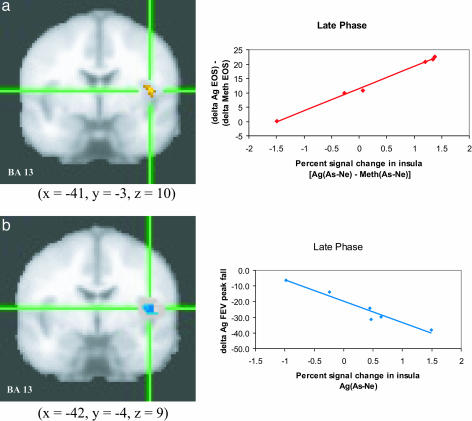
Functional Neuroimaging Measures. Contrary to predictions, no interactions or main effects for challenge were found. However, our individual differences analyses revealed that the increase in percentage of sputum EOS between the Ag versus Meth challenges correlated positively (r = 0.92, P < 0.01) with the corresponding Ag versus Meth difference for the As - Ne contrast in the ACC (Fig. 3a) for the late-phase scan session but not for the early phase scan session (r = -0.35, P = not significant). An equivalent positive correlation (r = 0.99, P < 0.001) was found in the left insula (Fig. 4a; early phase, r = 0.40, P = not significant). The direction of these correlations indicates that subjects with greater signal change in the ACC and insula in response to As versus Ne words, in the late-phase scan only,** show a larger increase in EOS in response to Ag versus Meth challenge. The same region of ACC showed a positive correlation (r = 0.98, P < 0.001), during the late-phase scan only (early phase, r = 0.66, P = not significant), between GC sensitivity of PBLs in response to Ag and Meth challenges and the corresponding difference in the As - Ne contrast (Fig. 3b). In other words, the more activity in this region was modulated, the less TNF-α production by PBLs was sensitive to suppression by DEX. During the Ag challenge, activity in the insula in response to As versus Ne words correlated negatively with the peak fall in FEV1 (Fig. 4b; late phase, r = -0.97, P < 0.01; early phase, r = -0.13, P = not significant), indicating that the more modulation in this region during the Ag challenge, the greater the decline in lung function.
Fig. 3.
Anterior cingulate cortex activity predicts peripheral measures of inflammatory potential. Percent signal change in the ACC in response to asthma compared with neutral words [As-Ne] and percentage of EOS during late phase antigen relative to methacholine challenge [Ag - Meth] (a)(r = 0.92, P < 0.01) and PBL production of TNF-α (% of production relative to no DEX) during late phase antigen relative to methacholine challenge [Ag - Meth] (b)(r = 0.98, P < 0.001). Note that clusters in a and b were identified independently and are not identical, although they largely overlap.
Fig. 4.
Insula activity predicts peripheral measures of inflammatory potential and lung function. Percent signal change in the left insula in response to As compared with Ne words [As - Ne] and percentage of EOS during late-phase antigen relative to methacholine challenge [Ag - Meth] (a)(r = 0.99, P < 0.001) and peak fall FEV1 during late phase antigen [Ag] challenge (b) (r = -0.97, P < 0.01). Note that clusters in a and b were identified independently and are not identical, although they largely overlap. For analyses with FEV1, only values for the Ag challenge were used because one participant was missing data from the Meth challenge.
Using a regression analysis, we determined that late-phase percent signal change in the ACC and insula (As - Ne contrast) during Ag challenge specifically, controlling for that during Meth challenge, accounted for a significant portion of variance, between 40% and 57%, in the peripheral measures (ACC/EOS: ΔR2 = 0.40, t (5) = 3.7, P < 0.05; ACC/TNF-α: ΔR2 = 0.53, t (5) = 9.3, P < 0.01; insula/EOS: ΔR2 = 0.57, t (5) = 18.1, P < 0.001). Moreover, the associations between both the insula with sputum EOS and the ACC with TNF-α were stronger during Ag than during Meth challenge (EOS: β = 0.76 vs. -0.59; TNF-α: β = 0.75 vs. -0.51).
To further determine the specificity of the brain-immune associations to asthma-relevant stimuli, we tested the associations between activity in these brain regions in response to negative compared with Ne words (Ag[Ng - Ne] - Meth[Ng - Ne]) and the peripheral measures. No significant association was present for either brain region with any of the peripheral measures (ACC/EOS: r = 0.00, P = 0.99; ACC/TNF-α: r = 0.2, P = 0.7; insula/EOS: r = 0.04, P = 0.95; insula/FEV1: r = -0.46, P = 0.36).
Discussion
By contrasting changes in brain activity in response to an Ag challenge to those of a Meth challenge, we were able to distinguish modulation caused by the general experience of airflow obstruction from that initiated by the onset of an inflammatory airway response. In addition, by contrasting brain modulation in response to As stimuli with that of Ne and negative stimuli, we were able to identify brain regions that may be hyperresponsive to contextually salient environmental cues. At this time, we do not know whether the signals we are observing in the brain represent the afferent modulation of neural activity by signaling from the lung or reflect central efferent processes that modulate the lung. What we do know is that the effects are highly specific to asthma content. The fact that they were also stronger in response to antigen suggests that it is the afferent signals associated with the development of inflammation that likely play the key role in modulating the sensitivity of the ACC and insula to the presentation of As stimuli. Indeed, these findings add functional significance to previous research that established a signaling route to alert the insula and ACC of the ongoing events in the lungs (3, 16) through pulmonary and chemoreceptor-containing afferent vagal fibers activated by proinflammatory cytokines.
In the present study, allergic asthmatic participants were selected because they developed an immediate and late-phase response to an allergen challenge. The events in the lungs of those who develop a late-phase response, including the increase in sputum EOS, are indistinguishable from those who do not during the early phase response to allergen (32). That is, sputum eosinophilia can follow Ag provocation independent of the pulmonary late-phase response, which suggests that the difference may originate outside of the airways. The magnitude of response of the insula and ACC to both the internal cues from the early phase response and salient external cues could be one factor in determining the extent of airway responsivity to inflammatory mediators and, subsequently, define who is a late-phase responder or more prone to experience airway obstruction and asthma symptoms.
Our data also indicate that PBLs of late-phase responders are less sensitive to suppression by GCs during an Ag challenge, resulting in greater production of TNF-α. GCs can inhibit inflammatory responses to prevent or reduce the injury after an immune reaction (33). Studies in both human asthmatics and animal models of asthma elegantly demonstrate this phenomenon by showing that inhibition of the inflammatory cascade, with administration of GCs before allergen exposure, results in the subsequent prevention of the late-phase response (4, 34). As in many diseases of inflammation, asthmatics show blunted GC release in response to stress (35), and some show reduced sensitivity to its immunosuppressive effects (36). A reduced GC response may lead to the enhancement of inflammatory processes in asthma or reflect a consequence of chronic inflammation. The capacity for GCs to attenuate the inflammatory response during an Ag challenge could represent another determinant of late-phase responder status, perhaps through modulation of proinflammatory cytokine-induced neural activation, and should be examined in future research.
In light of the relative nascence of this area of research, the interpretation of these findings should be measured, and replication is essential. The total number of participants is small, and, thus, only brain regions whose activity show very strong associations with peripheral physiological measures will be detected statistically. It is therefore likely that other brain regions, especially those involved in autonomic and neuroendocrine output, participate in this neural circuit but did not emerge in this study. Relatedly, our analyses were designed to compare neural responses to As and Ne word stimuli during differing physiological contexts and, thus, would not detect changes in brain activity that were constant across valence conditions. In addition, the small sample size precludes interpretation of the absolute magnitude of the individual measures, because the range of values and number of individuals at each level is very limited.
With appropriate caution taken, however, these data may have broader implications for the role of the CNS in the regulation and dysregulation of inflammation. They represent a previously undescribed approach to understanding the functional link between emotion processing circuits in the brain and peripheral physiological processes relevant to disease progression. We do not know whether the increased responsivity of the brain regions highlighted in this study reflects processes specific to asthma or a more general phenomenon involved in monitoring homeostatic processes such as inflammation, depending on the emotional and cognitive context. Although a more general phenomenon that applies to other stress-related disorders seems likely, our data underscore the fact that the signals from the periphery sensitize the insula and ACC to stimuli that are specific to the disorder in question. Whether the mechanism for this specificity depends on where the peripheral signals are generated or the specific molecule responsible for the signaling is not known at this time. Most importantly, these data underscore the importance of specific CNS circuitry in the monitoring and regulation of peripheral disorders and suggest potential new targets for the development of therapeutic interventions in stress-responsive biomedical disorders.
Supplementary Material
Acknowledgments
This research was supported by grants from the MacArthur Foundation Mind, Brain, Body, and Health Initiative and the Fetzer Foundation (to R.J.D., W.W.B., and J.F.S.), and National Institute of Mental Health Grants P50-MH52354 and P50-MH61083 (to R.J.D.) and National Institutes of Health Grant P50-HL56396 (to W.W.B.).
Author contributions: M.A.R., W.W.B., T.J., C.A.S., and R.J.D. designed research; M.A.R., G.M.C., M.M.J., J.A.B., and J.F.S. performed research; J.A.B. and J.F.S. contributed new reagents/analytic tools; M.A.R., T.J., C.A.S., G.M.C., J.A.B., and J.F.S. analyzed data; and M.A.R., W.W.B., and R.J.D. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACC, anterior cingulate cortex; Ag, antigen; As, asthma-relevant; DEX, dexamethasone; EOS, eosinophils; FEV1, forced expiratory volume in 1 second; GC, glucocorticoids; Meth, methacholine; Ne, valence-neutral; Ng, negative; PBL, peripheral blood leukocyte.
Data deposition: The fMRI data have been deposited in the fMRI Data Center, www.fmridc.org (accession no. 2-2005-119R1).
Footnotes
During the Ag challenge, lung function had not significantly declined at the 4h scan (Ag vs. Meth; t(5) = 1.8, p >.1). However, lung function had begun to decline at 4h, reflecting the onset of the late phase response and inflammation. The peak in fall of FEV1 occurred between 6–8 h after challenge for every participant.
References
- 1.Lehrer, P. M., Isenberg, S. & Hochron, S. M. (1993) J. Asthma 30, 5-21. [DOI] [PubMed] [Google Scholar]
- 2.Liu, L. Y., Coe, C. L., Swenson, C. A., Kelly, E. A., Kita, H. & Busse, W. W. (2002) Am. J. Respir. Crit. Care Med. 165, 1062-1067. [DOI] [PubMed] [Google Scholar]
- 3.Matalka, K. Z. (2003) Neuroendocrinol. Lett. 24, 283-292.14646999 [Google Scholar]
- 4.Cieslewicz, G. Cieslewicz, G., Tomkinson, A., Adler, A., Duez, C, Schwarze, J., Takeda, K., Larson, K. A., Lee, J. J., Irvin, C. G. & Gelfand, E. W. (1999) J. Clin. Invest. 104, 301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maier, S. F. & Watkins, L. R. (1998) Psychol. Rev. 105, 83-107. [DOI] [PubMed] [Google Scholar]
- 6.Shanks, N., Harbuz, M. S., Jessop, D. S., Perks, P., Moore, P. M. & Lightman, S. L. (1998) Ann. N.Y. Acad. Sci. 840, 599-607. [DOI] [PubMed] [Google Scholar]
- 7.Black, P. H. (2002) Brain Behav. Immun. 16, 622-653. [DOI] [PubMed] [Google Scholar]
- 8.Donahue, R. R., LaGraize, S. C. & Fuchs, P. N. (2001) Brain Res. 897, 131-138. [DOI] [PubMed] [Google Scholar]
- 9.Maier, S. F. (2003) Brain Behav. Immun. 17, 69-85. [DOI] [PubMed] [Google Scholar]
- 10.Wichers, M. & Maes, M. J. (2003) Neuropsychopharmacology 5, 375-388. [DOI] [PubMed] [Google Scholar]
- 11.Bluthé, R. M., Pawlowski, M., Suarez, S. Parnet, P., Pittman, Q., Kelley, K. W. & Dantzer R. (1994) Psychoneuroendocrinology 19, 197-207. [DOI] [PubMed] [Google Scholar]
- 12.Craig, A. D. (2002) Nat. Rev. Neurosci. 3, 655-666. [DOI] [PubMed] [Google Scholar]
- 13.Banzett, R. B., Mulnier, H. E., Murphy, K., Rosen, S. D., Wise, R. J. & Adams, L. (2000) NeuroReport 11, 2117-2120. [DOI] [PubMed] [Google Scholar]
- 14.Liotti, M., Brannan, S., Egan, G., Shade, R., Madden, L., Abplanalp, B., Robillard, R., Lancaster, J., Zamarripa, F. E., Fox, P. T. & Denton, D. (2001) Proc. Natl. Acad. Sci. USA 98, 2035-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasui, Y., Breder, C. D., Saper, C. B. & Cechetto, D. F. (1991) J. Comp. Neurol. 303, 355-374. [DOI] [PubMed] [Google Scholar]
- 16.Devinsky, O., Morrell, M. J. & Vogt, B. A. (1995) Brain 118, 279-306. [DOI] [PubMed] [Google Scholar]
- 17.Greenspan, J. D., Lee, R. R. & Lenz, F. A. (1999) Pain 81, 273-282. [DOI] [PubMed] [Google Scholar]
- 18.Craig, A. D., Chen, K., Bandy, D. & Reiman, E. M. (2000) Nature Neurosci. 3, 184-190. [DOI] [PubMed] [Google Scholar]
- 19.Critchley, H. D., Wiens, S., Rotshtein, P., Öhman, A. & Dolan, R. J. (2004) Nat. Neurosci. 7, 189-195. [DOI] [PubMed] [Google Scholar]
- 20.Rainville, P. Duncan, G. H., Price, D. D., Carrier, B. & Bushnell, M. C. (1997) Science 277, 968-971. [DOI] [PubMed] [Google Scholar]
- 21.Hurt, R. W. & Ballentine, H. T. J. (1974) Clin. Neurosurg. 21, 334-351. [DOI] [PubMed] [Google Scholar]
- 22.Critchley, H. D., Mathias, C. J., Josephs, O., O'Dougherty, J., Zanini, S., Dewer, B.-K., Cipolotti, L., Shallice, T. & Dolan, R. J. (2003) Brain 126, 2139-2152. [DOI] [PubMed] [Google Scholar]
- 23.Sternberg, E. M., Hill, J. M., Chrousos, G. P., Kamilaris, T., Listwak, S. J., Gold, P. W. & Wilder, R. L. (1989) Proc. Natl. Acad. Sci. USA 86, 2374-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, G. E., Cohen, S. & Ritchey, A. K. (2002) Health Psychol. 21, 531-541. [DOI] [PubMed] [Google Scholar]
- 25.Stark, J. L., Avitsur, R., Hunzeker, J., Padgett, D. A. & Sheridan J. F. (2002) J. Neuroimmunol. 124, 9-15. [DOI] [PubMed] [Google Scholar]
- 26.DeRijk, R., Petrides, J., Deuster, P., Gold, P. W. & Sternberg, E. M. (1996) J. Clin. Endocrinol. Metab. 81, 228-235. [DOI] [PubMed] [Google Scholar]
- 27.Anonymous (1995) Am. J. Respir. Crit. Care Med. 152, 1107-1136. [DOI] [PubMed] [Google Scholar]
- 28.Liu, L. Y, Swenson, C. A., Kita, H., Kelly, E. A. B. & Busse, W. W. (2000) J. Allergy Clin. Immunol. 106, 1063-1069. [DOI] [PubMed] [Google Scholar]
- 29.DeRijk, R., Michelson, D., Karp, B., Petrides, J., Galliven, E., Deuster, P., Paciotti, G., Gold, P. W. & Sternberg, E. M. (1997) J. Clin. Endocrinol. Metab. 82, 2182-2191. [DOI] [PubMed] [Google Scholar]
- 30.Bradley, M. M. & Lang, P. J. (1999) Affective Norms for English Words (ANEW): Stimuli, Instruction Manual, and Affective Ratings (Univ. of Florida, Gainesville), Technical Report C-1.
- 31.Cox, R. W. (1996) Comput. Biomed. Res. 29, 162-173. [DOI] [PubMed] [Google Scholar]
- 32.Liu, L. Y. Swenson, C. A., Kelly, E. A., Kita, H., Jarjour, N. N. & Busse, W. W. (2003) J. Allergy Clin. Immunol. 111, 818-825. [DOI] [PubMed] [Google Scholar]
- 33.Perretti, M. & Ahluwalia, A. (2000) Microcirculation 7, 147-161. [PubMed] [Google Scholar]
- 34.Kidney, J. C., Boulet, L.-P., Hargreave, F. E., Deschesnes, F., Swystun, V. A., O'Byrne, P. M., Choudry, N., Morris, M. M., Jennings, B., Andersson, N., et al. (1997) J. Allergy Clin. Immunol. 100, 65-70. [DOI] [PubMed] [Google Scholar]
- 35.Buske-Kirschbaum, A., von Auer, K., Krieger, S., Weis, S., Rauh, W. & Hellhammer, D. (2003) Psychosom. Med. 65, 806-810. [DOI] [PubMed] [Google Scholar]
- 36.Leung, D. Y. M. & Bloom, J. W. (2003) J. Allergy Clin. Immunol. 111, 3-22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.