Abstract
1. The quantal release of neurotransmitter and the fine structure of frog neuromuscular junctions has been examined in the presence of the xanthene dye Erythrosin B.
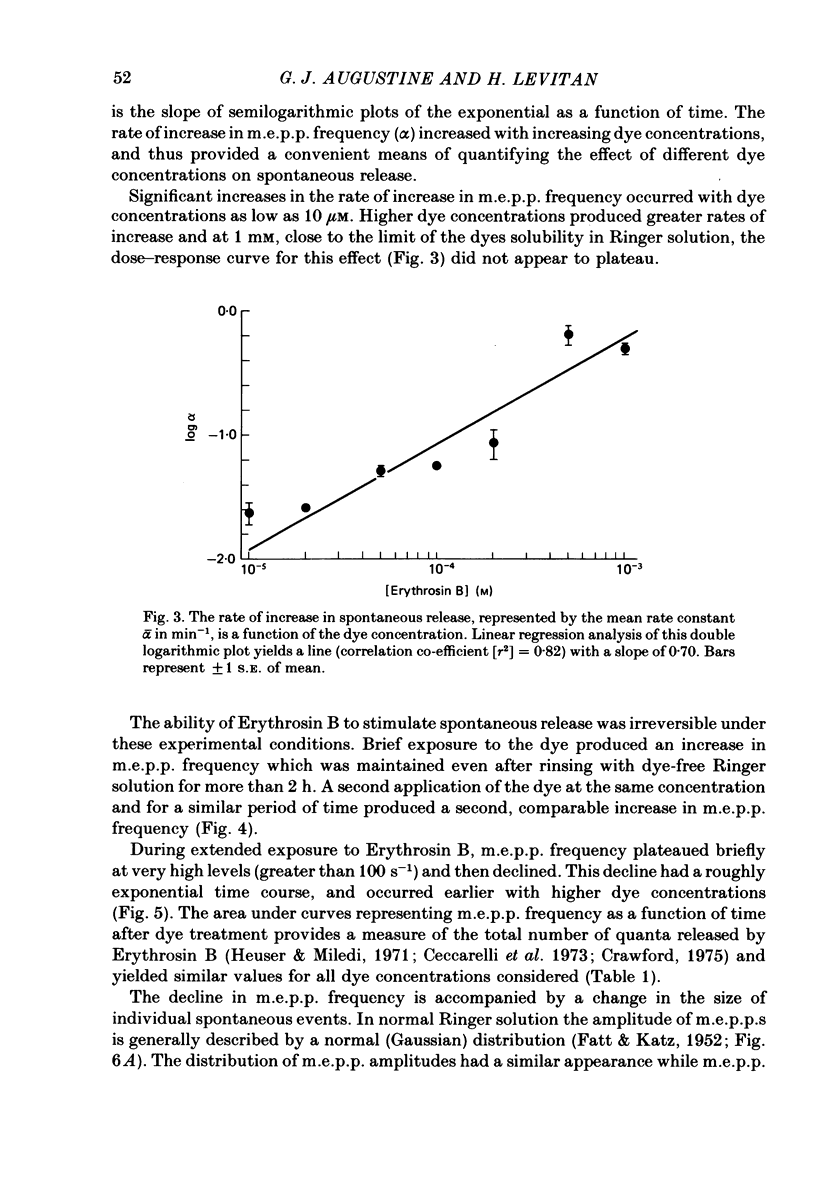
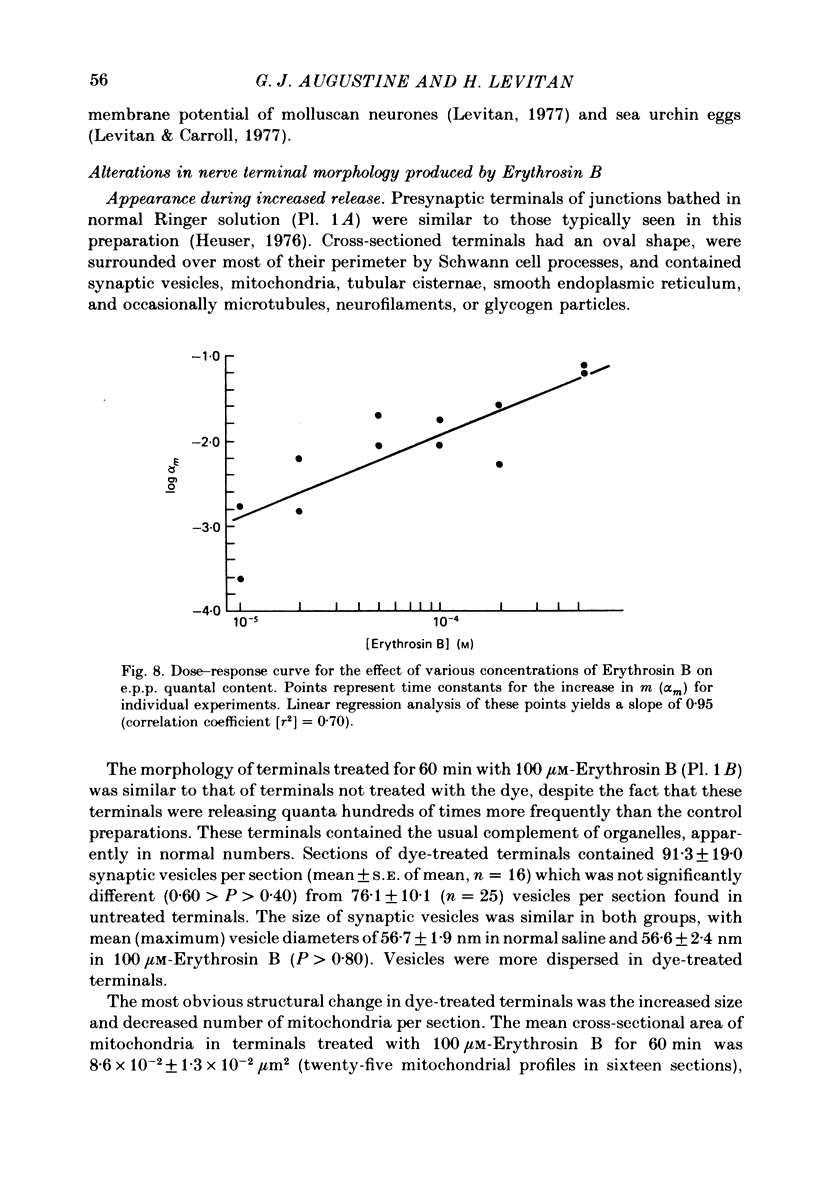
2. At concentrations of 10 μM or greater, Erythrosin B produced time- and dose-dependent increases in transmitter release from presynaptic nerve terminals.
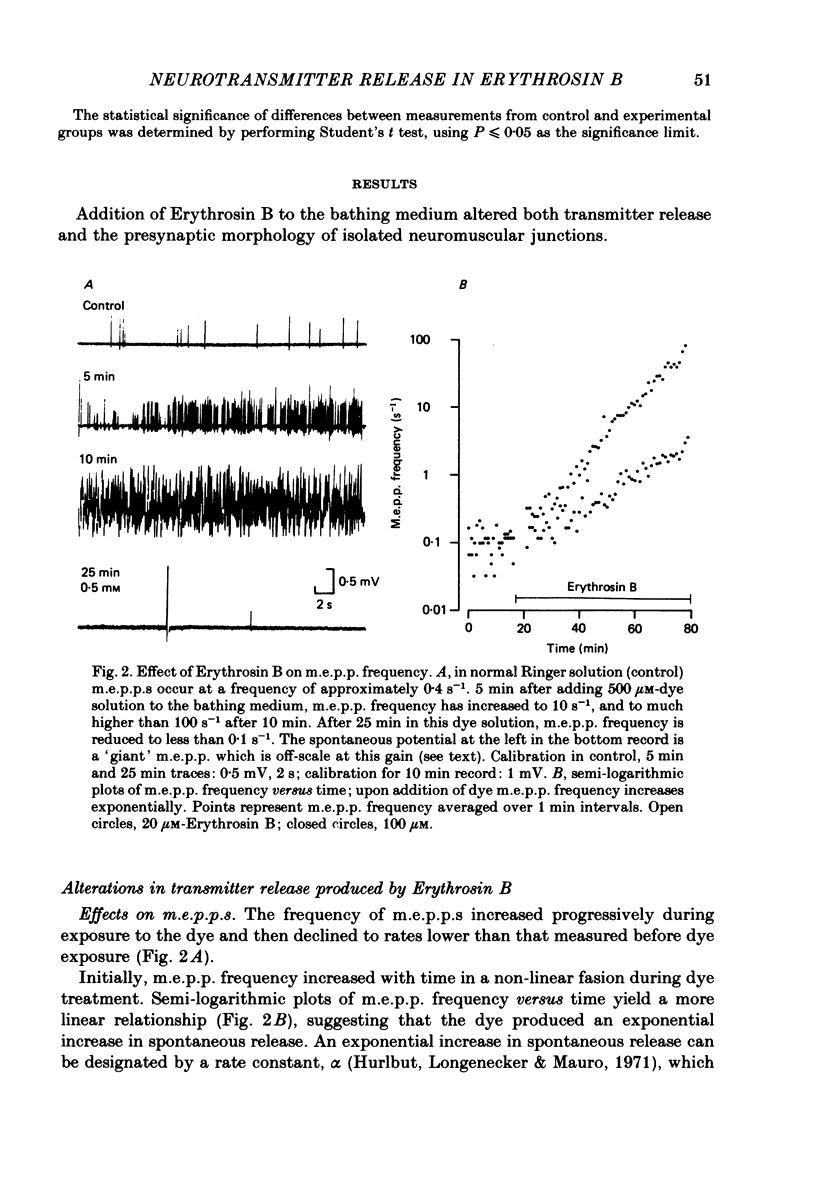
3. Miniature end-plate potential (m.e.p.p.) frequency increased in an exponential manner during continuous exposure to the dye. The rate constant for this exponential was dose-dependent, increasing with concentrations from 10 μM to 1 mM.
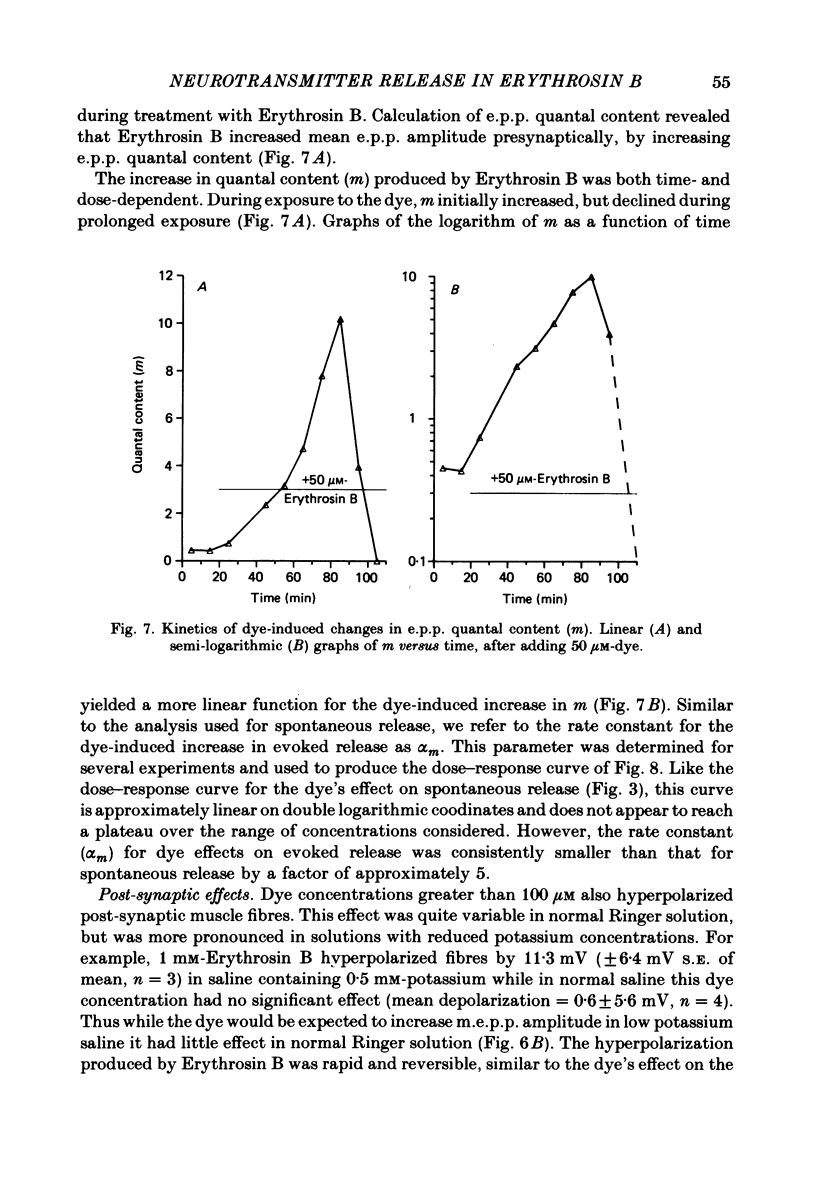
4. The amplitude of evoked end-plate potentials (e.p.p.s) also increased exponentially during dye treatment, primarily due to an increase in quantal content. Rate constants for this effect were also dose-dependent, and were approximately 1/5 as large as those for m.e.p.p.s.
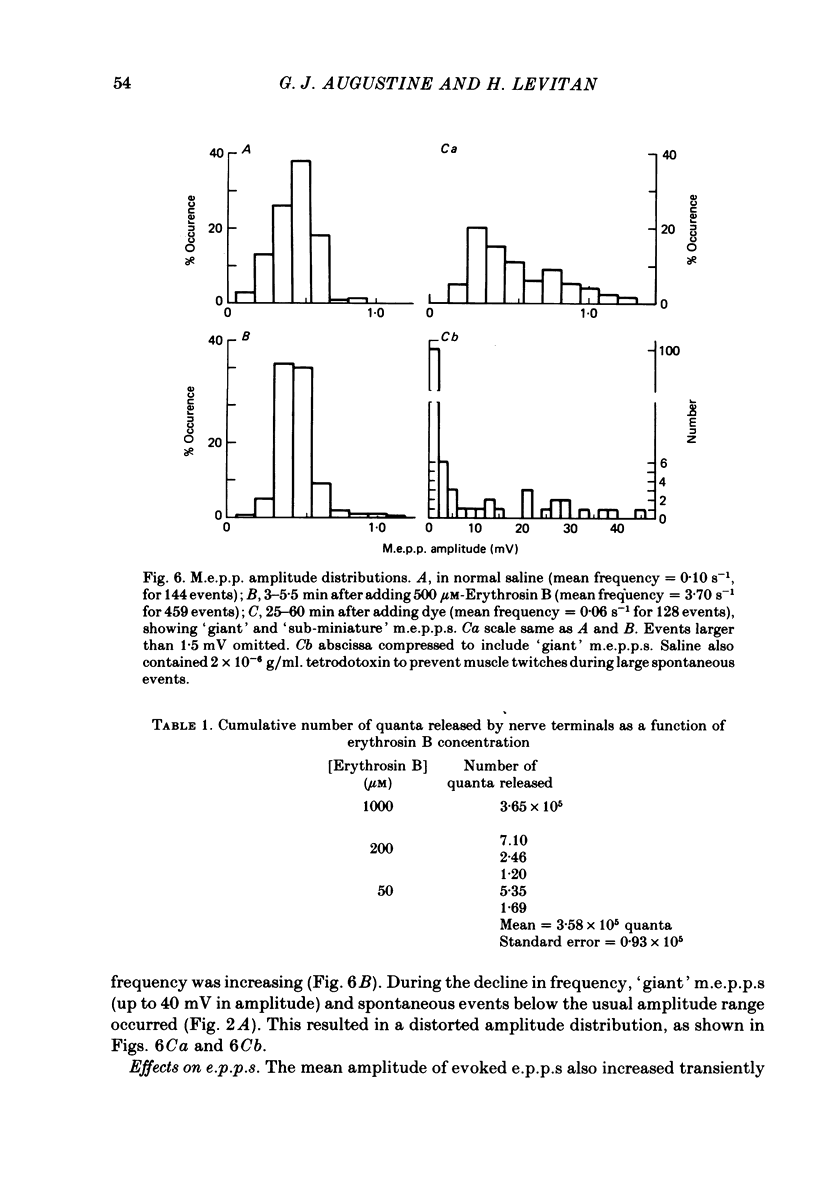
5. While the frequency of m.e.p.p.s was increasing, their amplitude distribution did not qualitatively change. Thus the dye has little effect on the size of individual quanta.
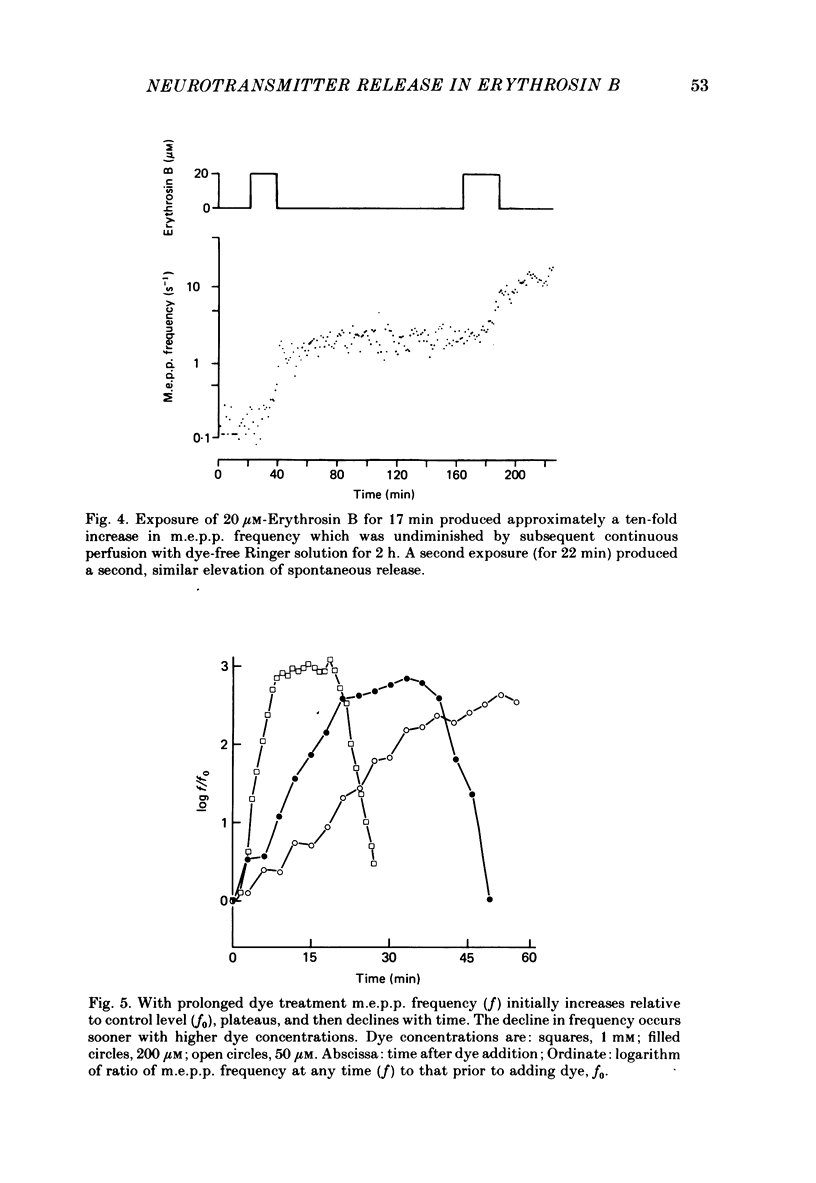
6. The presynaptic effects of Erythrosin B were irreversible under these experimental conditions. Brief exposure to the dye caused increases in m.e.p.p. frequency and e.p.p. amplitude which were maintained at steady levels during extensive rinsing with dye-free Ringer solution.
7. Prolonged exposure to the dye caused an eventual decrease in m.e.p.p. frequency and abolition of e.p.p.s. Coincident with this decline `giant' m.e.p.p.s as large as 40 mV were observed.
8. At dye concentrations greater than approximately 200 μM, Erythrosin B rapidly and reversibly increased the membrane potential and input resistance of muscle fibres. This post-synaptic effect was small and variable in normal saline, but was pronounced in low potassium solutions.
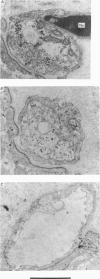
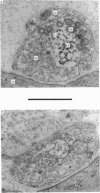
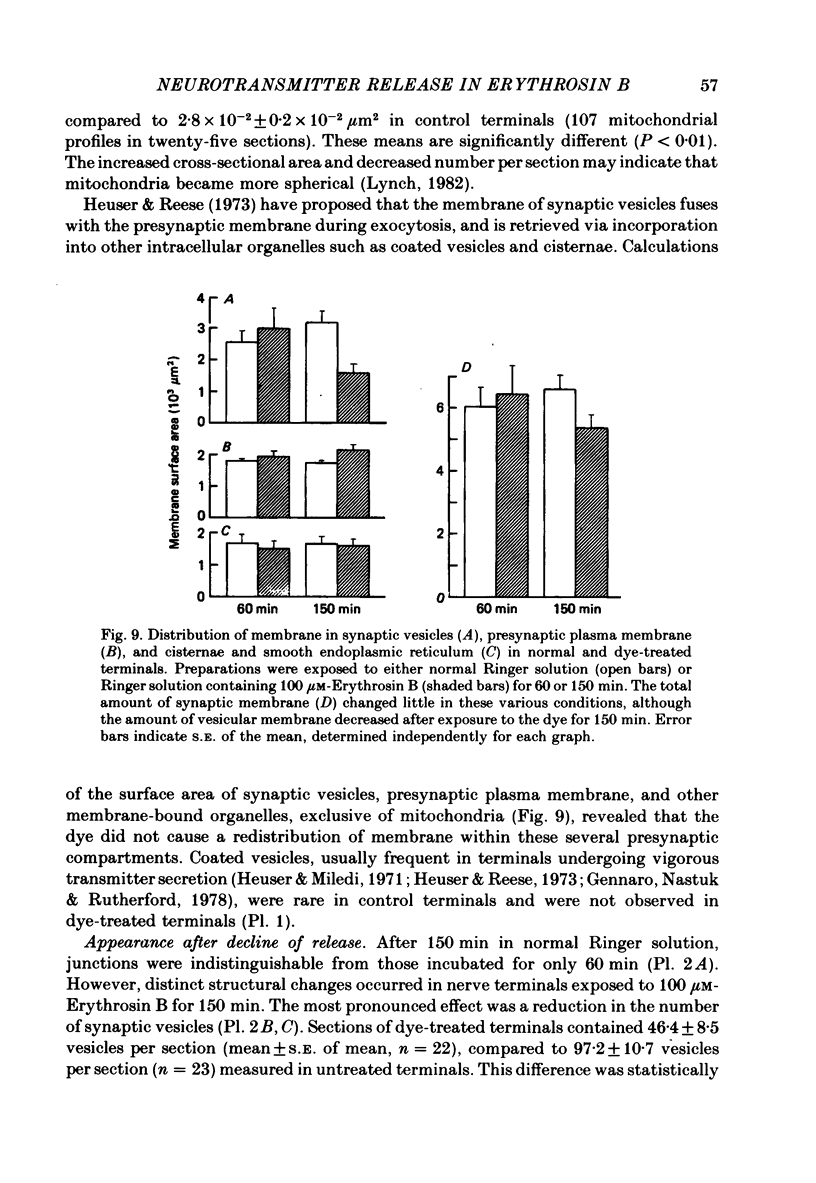
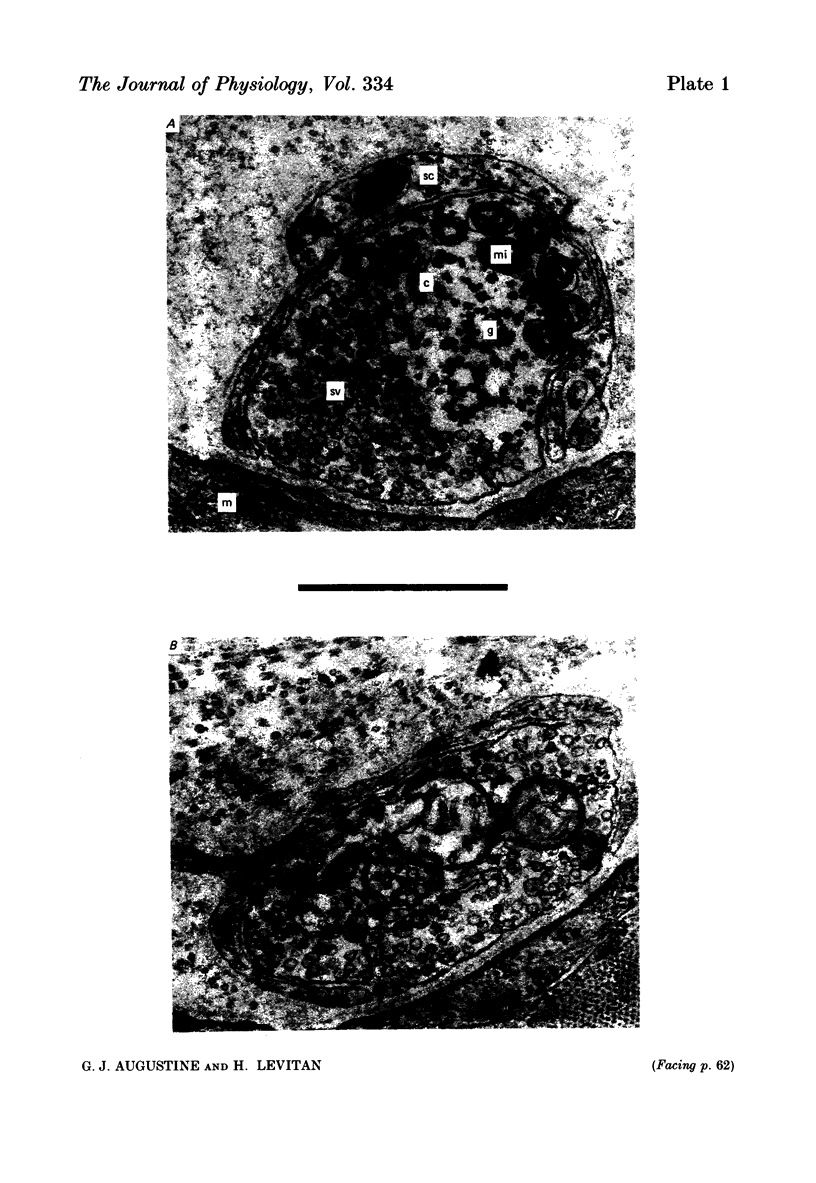
9. During the period that release was enhanced by Erythrosin B, presynaptic nerve terminals contained the normal complement of synaptic vesicles and other organelles. Mitochondria were swollen in this condition.
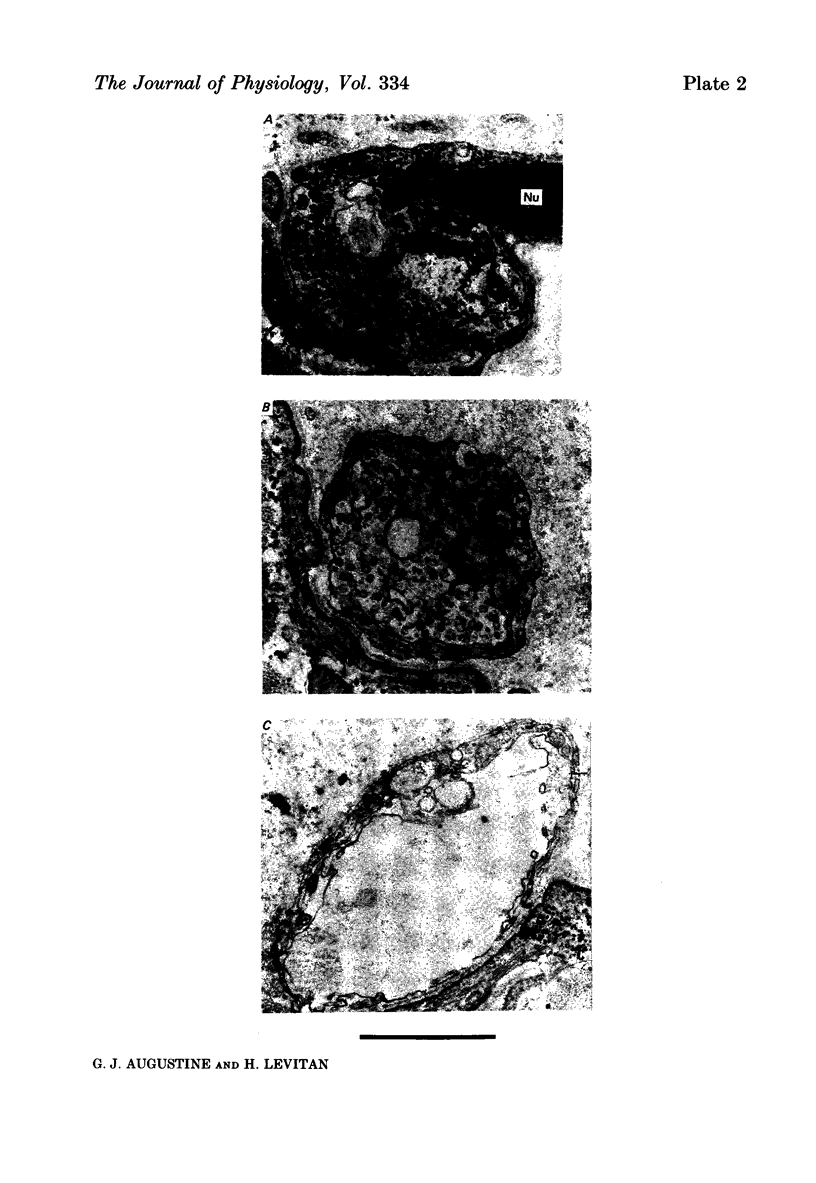
10. After m.e.p.p. frequency declined below normal levels and `giant' m.e.p.p.s appeared, the number of synaptic vesicles within nerve terminals declined and dilated cisternae were present. Mitochondria were swollen further.
11. These results do not reveal any mechanism to explain the ability of Erythrosin B to increase transmitter release, but the decline in release may be caused by partial depletion of synaptic vesicles. The `giant' m.e.p.p.s could be due to the discharge of acetylcholine from cisternae.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Jr, Levitan H. Neurotransmitter release from a vertebrate neuromuscular synapse affected by a food dye. Science. 1980 Mar 28;207(4438):1489–1490. doi: 10.1126/science.6244619. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Levitan H. Presynaptic effect of Erythrosin B at the frog neuromuscular junction: ion and photon sensitivity. J Physiol. 1983 Jan;334:65–77. doi: 10.1113/jphysiol.1983.sp014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKS R., HUXLEY H. E., KATZ B. The fine structure of the neuromuscular junction of the frog. J Physiol. 1960 Jan;150:134–144. doi: 10.1113/jphysiol.1960.sp006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyne A. F. Neurosecretion: integration of recent findings into the vesicle hypothesis. Life Sci. 1978 Jun 19;22(23):2057–2066. doi: 10.1016/0024-3205(78)90448-4. [DOI] [PubMed] [Google Scholar]
- Carroll E. J., Jr, Levitan H. Fertilization in the sea urchin, Strongylocentrotus purpuratus is blocked by fluorescein dyes. Dev Biol. 1978 Apr;63(2):432–440. doi: 10.1016/0012-1606(78)90147-1. [DOI] [PubMed] [Google Scholar]
- Castillo J. D., Pumplin D. W. Discrete and discontinuous action of brown widow spider venom on the presynaptic nerve terminals of frog muscle. J Physiol. 1975 Nov;252(2):491–508. doi: 10.1113/jphysiol.1975.sp011154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P., Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Su M. J., Lee J. D., Eaker D. Effects of Sr2+ and Mg2+ on the phospholipase A and the presynaptic neuromuscular blocking actions of beta-bungarotoxin, crotoxin and taipoxin. Naunyn Schmiedebergs Arch Pharmacol. 1977 Sep;299(2):155–161. doi: 10.1007/BF00498557. [DOI] [PubMed] [Google Scholar]
- Crawford A. C. Lithium ions and the release of transmitter at the frog neuromuscular junction. J Physiol. 1975 Mar;246(1):109–142. doi: 10.1113/jphysiol.1975.sp010882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W., Tonge D. A. The effects of tetanus toxin on neuromuscular transmission and on the morphology of motor end-plates in slow and fast skeletal muscle of the mouse. J Physiol. 1973 Jan;228(1):157–172. doi: 10.1113/jphysiol.1973.sp010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Fritz L. C., Atwood H. L., Jahromi S. S. Lobster neuromuscular junctions treated with black widow spider venom: correlation between ultrastructure and physiology. J Neurocytol. 1980 Oct;9(5):699–721. doi: 10.1007/BF01205034. [DOI] [PubMed] [Google Scholar]
- Gage P. W. The effect of methyl, ethyl and n-propyl alcohol on neuromuscular transmission in the rat. J Pharmacol Exp Ther. 1965 Nov;150(2):236–243. [PubMed] [Google Scholar]
- Gennaro J. F., Jr, Nastuk W. L., Rutherford D. T. Reversible depletion of synaptic vesicles induced by application of high external potassium to the frog neuromuscular junction. J Physiol. 1978 Jul;280:237–247. doi: 10.1113/jphysiol.1978.sp012382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Miledi R. The effect of type D botulinum toxin on frog neuromuscular junctions. J Physiol. 1971 Sep;217(2):497–515. doi: 10.1113/jphysiol.1971.sp009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart M. P., Reese T. S., Brinley F. J., Jr Endoplasmic reticulum sequesters calcium in the squid giant axon. Science. 1978 Dec 22;202(4374):1300–1303. doi: 10.1126/science.725607. [DOI] [PubMed] [Google Scholar]
- Heuser J. E. Proceedings: A possible origin of the 'giant' spontaneous potentials that occur after prolonged transmitter release at frog neuromuscular junctions. J Physiol. 1974 Jun;239(2):106P–108P. doi: 10.1113/jphysiol.1974.sp010593. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Howard B. D., Gundersen C. B., Jr Effects and mechanisms of polypeptide neurotoxins that act presynaptically. Annu Rev Pharmacol Toxicol. 1980;20:307–336. doi: 10.1146/annurev.pa.20.040180.001515. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Southard J. H. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976 Aug 25;251(16):5069–5077. [PubMed] [Google Scholar]
- Hurlbut W. P., Longenecker H. B., Jr, Mauro A. Effects of calcium and magnesium on the frequency of miniature end-plate potentials during prolonged tetanization. J Physiol. 1971 Dec;219(1):17–38. doi: 10.1113/jphysiol.1971.sp009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. F., Kwanbunbumpen S. The effects of nerve stimulation and hemicholinium on synaptic vesicles at the mammalian euromuscular junction. J Physiol. 1970 Mar;207(1):31–50. doi: 10.1113/jphysiol.1970.sp009046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Failure of neuromuscular propagation in rats. J Physiol. 1958 Mar 11;140(3):440–461. [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Estimates of quantal content during 'chemical potentiation' of transmitter release. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol. 1969 Aug;203(3):689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B., Deutsch J. W., Carlson S. S., Wagner J. A. Biochemistry of neurotransmitter release. Annu Rev Neurosci. 1979;2:399–446. doi: 10.1146/annurev.ne.02.030179.002151. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Oberg S. G., Strong P. N., Wagner G. M. beta-Bungarotoxin, a phospholipase that stimulates transmitter release. Cold Spring Harb Symp Quant Biol. 1976;40:117–125. doi: 10.1101/sqb.1976.040.01.013. [DOI] [PubMed] [Google Scholar]
- Kita H., Van Der Kloot W. Effects of the ionophore X-537A on acetylcholine release at the frog neuromuscular junction. J Physiol. 1976 Jul;259(1):177–198. doi: 10.1113/jphysiol.1976.sp011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Gross C. E. Multimodal distribution of frog miniature endplate potentials in adult denervated and tadpole leg muscle. J Gen Physiol. 1974 Jul;64(1):85–103. doi: 10.1085/jgp.64.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Llados F., Matteson D. R. Spontaneous subminature end-plate potentials in mouse diaphragm muscle: evidence for synchronous release. J Physiol. 1976 Nov;262(3):553–581. doi: 10.1113/jphysiol.1976.sp011610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinsky M. S., Morrison-Graham K. Structure of developing frog neuromuscular junctions. J Neurocytol. 1980 Jun;9(3):321–342. doi: 10.1007/BF01181540. [DOI] [PubMed] [Google Scholar]
- Levitan H. Food, drug, and cosmetic dyes: biological effects related to lipid solubility. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2914–2918. doi: 10.1073/pnas.74.7.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R. Depolarization-release coupling systems in neurons. Neurosci Res Program Bull. 1977 Dec;15(4):555–687. [PubMed] [Google Scholar]
- Logan W. J., Swanson J. M. Erythrosin B inhibition of neurotransmitter accumulation by rat brain homogenate. Science. 1979 Oct 19;206(4416):363–364. doi: 10.1126/science.39341. [DOI] [PubMed] [Google Scholar]
- Longenecker H. E., Jr, Hurlbut W. P., Mauro A., Clark A. W. Effects of black widow spider venom on the frog neuromuscular junction. Effects on end-plate potential, miniature end-plate potential and nerve terminal spike. Nature. 1970 Feb 21;225(5234):701–703. doi: 10.1038/225701a0. [DOI] [PubMed] [Google Scholar]
- Lynch K. The effects of chronic stimulation on the morphology of the frog neuromuscular junction. J Neurocytol. 1982 Feb;11(1):81–107. doi: 10.1007/BF01258006. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Miller D. C. Is the quantum of transmitter release composed of subunits? A critical analysis in the mouse and frog. J Physiol. 1981 Feb;311:267–287. doi: 10.1113/jphysiol.1981.sp013584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaranche R., Thieffry M., Israel M. Effect of the venom of Glycera convoluta on the spontaneous quantal release of transmitter. J Cell Biol. 1980 May;85(2):446–458. doi: 10.1083/jcb.85.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P. N. The composition of Erythrosins, Fluorescein, Phloxine and Rose Bengal: a study using thin-layer chromatography and solvent extraction. Histochem J. 1976 Sep;8(5):487–499. doi: 10.1007/BF01003838. [DOI] [PubMed] [Google Scholar]
- McGraw C. F., Somlyo A. V., Blaustein M. P. Localization of calcium in presynaptic nerve terminals. An ultrastructural and electron microprobe analysis. J Cell Biol. 1980 May;85(2):228–241. doi: 10.1083/jcb.85.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Ornberg R. L., Reese T. S. A freeze-substitution method for localizing divalent cations: examples from secretory systems. Fed Proc. 1980 Aug;39(10):2802–2808. [PubMed] [Google Scholar]
- Oxford G. S., Pooler J. P., Narahashi T. Internal and external application of photodynamic sensitizers on squid giant axons. J Membr Biol. 1977 Sep 14;36(2-3):159–173. doi: 10.1007/BF01868149. [DOI] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S. Action of brown widow spider venom and botulinum toxin on the frog neuromuscular junction examined with the freeze-fracture technique. J Physiol. 1977 Dec;273(2):443–457. doi: 10.1113/jphysiol.1977.sp012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pécot-Dechavassine M. Action of vinblastine on the spontaneous release of acetylcholine at the frog neuromuscular junction. J Physiol. 1976 Sep;261(1):31–48. doi: 10.1113/jphysiol.1976.sp011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pécot-Dechavassine M., Couteaux R. Modifications structurales des terminaisons motrices de muscles de grenouille soumis á l'action de la vinblastine. C R Acad Sci Hebd Seances Acad Sci D. 1975 Mar 3;280(9):1099–1101. [PubMed] [Google Scholar]
- Rahamimoff R., Lev-Tov A., Meiri H. Primary and secondary regulation of quantal transmitter release: calcium and sodium. J Exp Biol. 1980 Dec;89:5–18. doi: 10.1242/jeb.89.1.5. [DOI] [PubMed] [Google Scholar]
- Sato T., Shamoto M. A simple rapid polychrome stain for epoxy-embedded tissue. Stain Technol. 1973 Sep;48(5):223–227. doi: 10.3109/10520297309116628. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the role of barium in supporting the asynchronous release of acetylcholine quanta by motor nerve impulses. J Physiol. 1978 Jan;274:157–171. doi: 10.1113/jphysiol.1978.sp012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. E., Clark A. W., Kuster T. A. Suppression by elevated calcium of black widow spider venom activity at frog neuromuscular junctions. J Neurocytol. 1977 Oct;6(5):519–539. doi: 10.1007/BF01205217. [DOI] [PubMed] [Google Scholar]
- Smith J. E., Reese T. S. Use of aldehyde fixatives to determine the rate of synaptic transmitter release. J Exp Biol. 1980 Dec;89:19–29. doi: 10.1242/jeb.89.1.19. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]