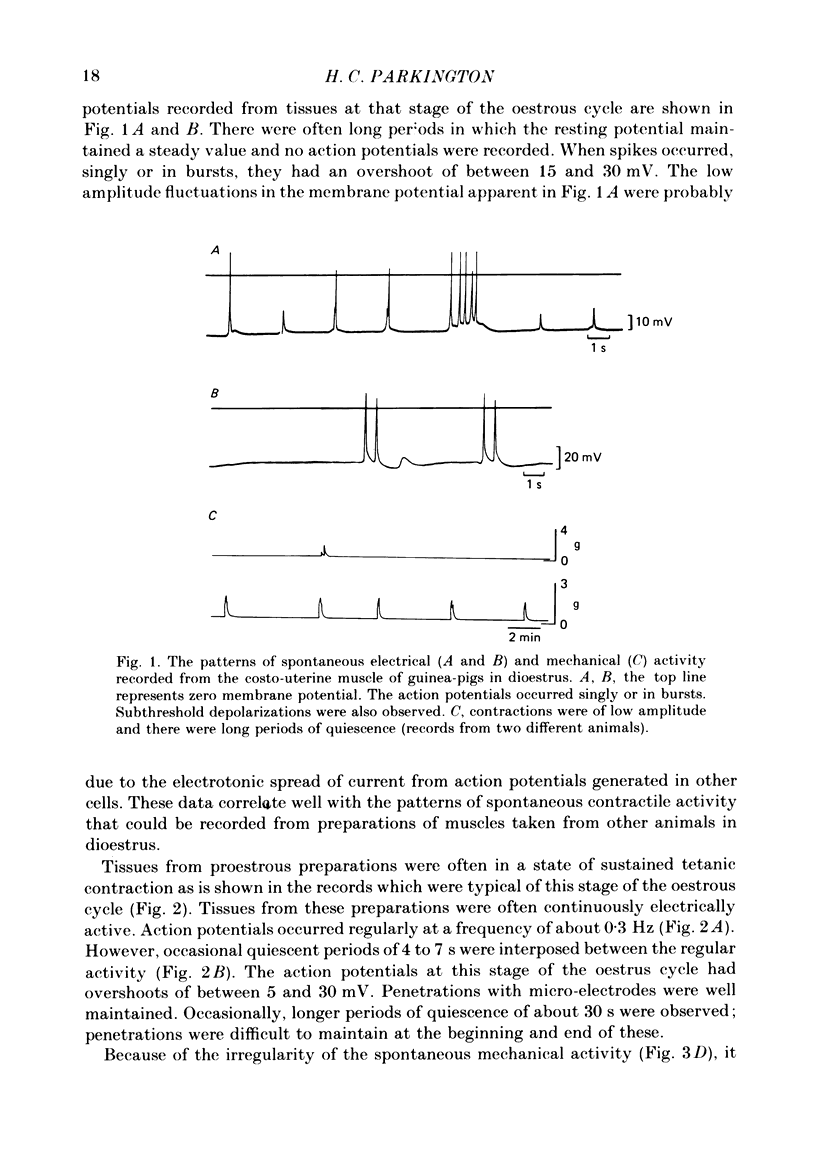
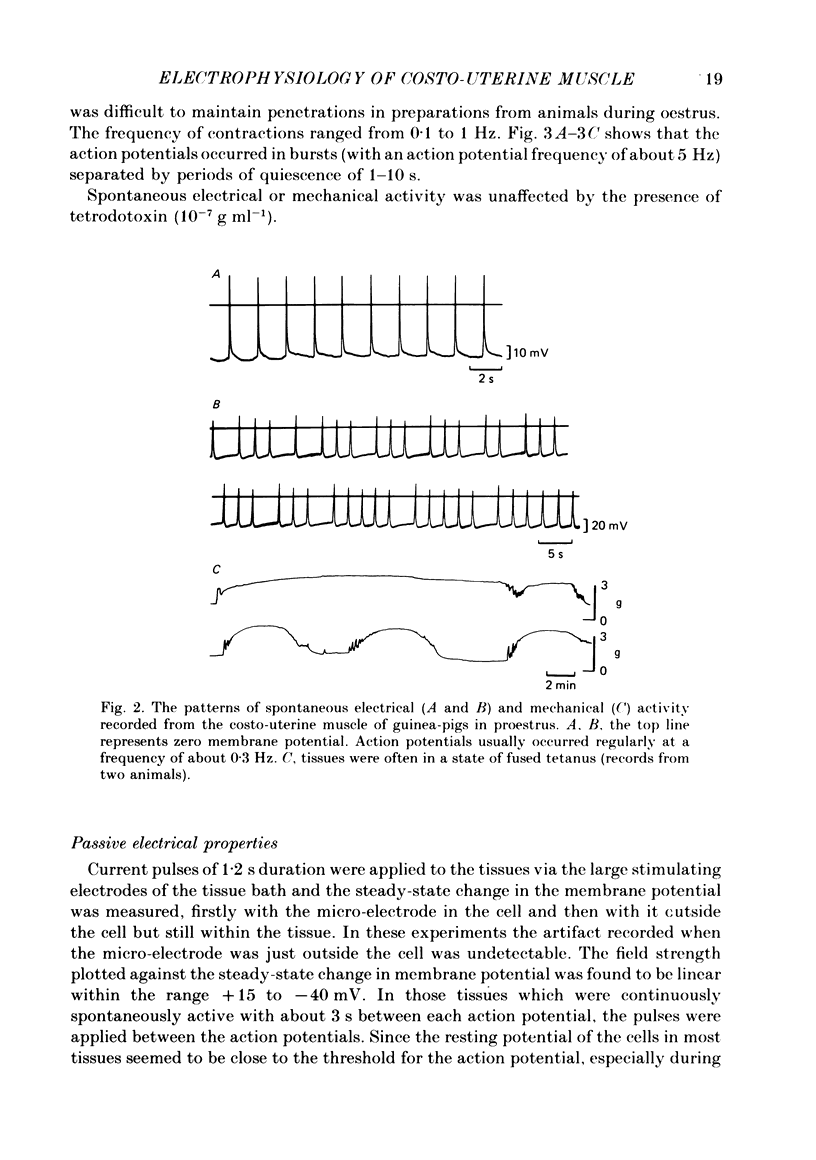
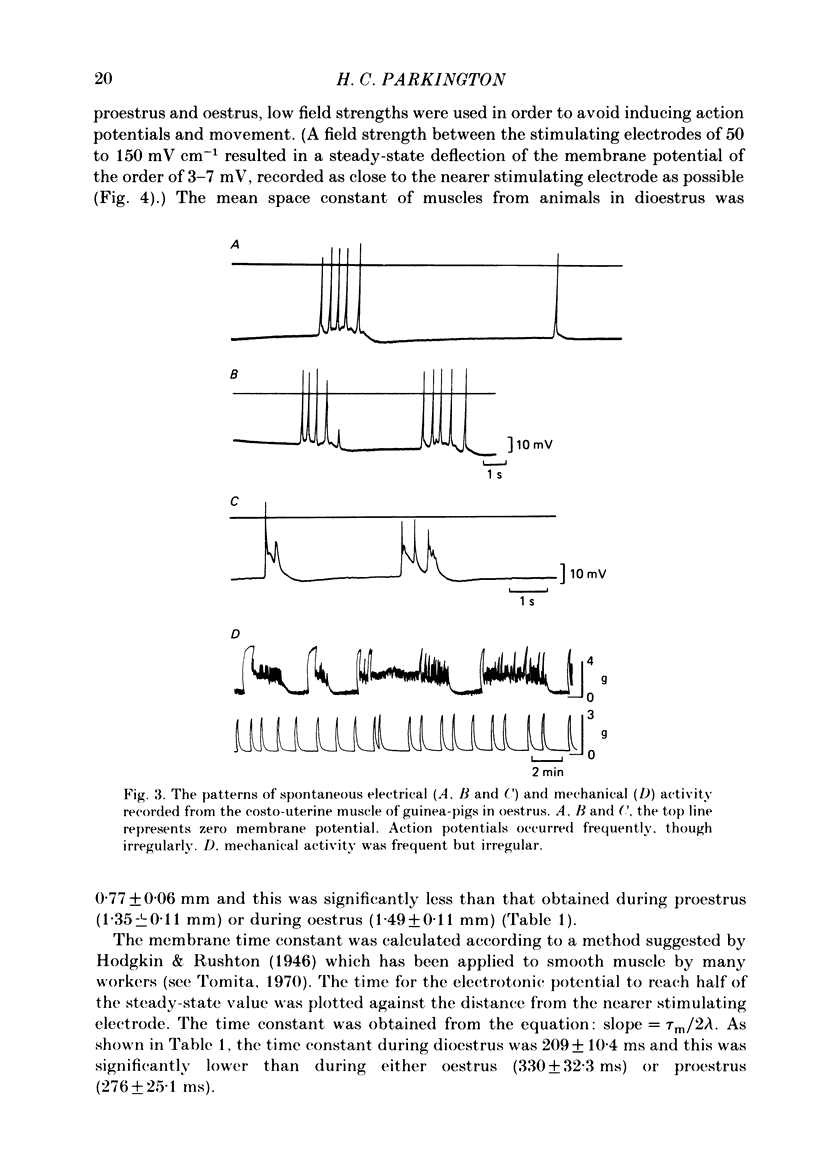
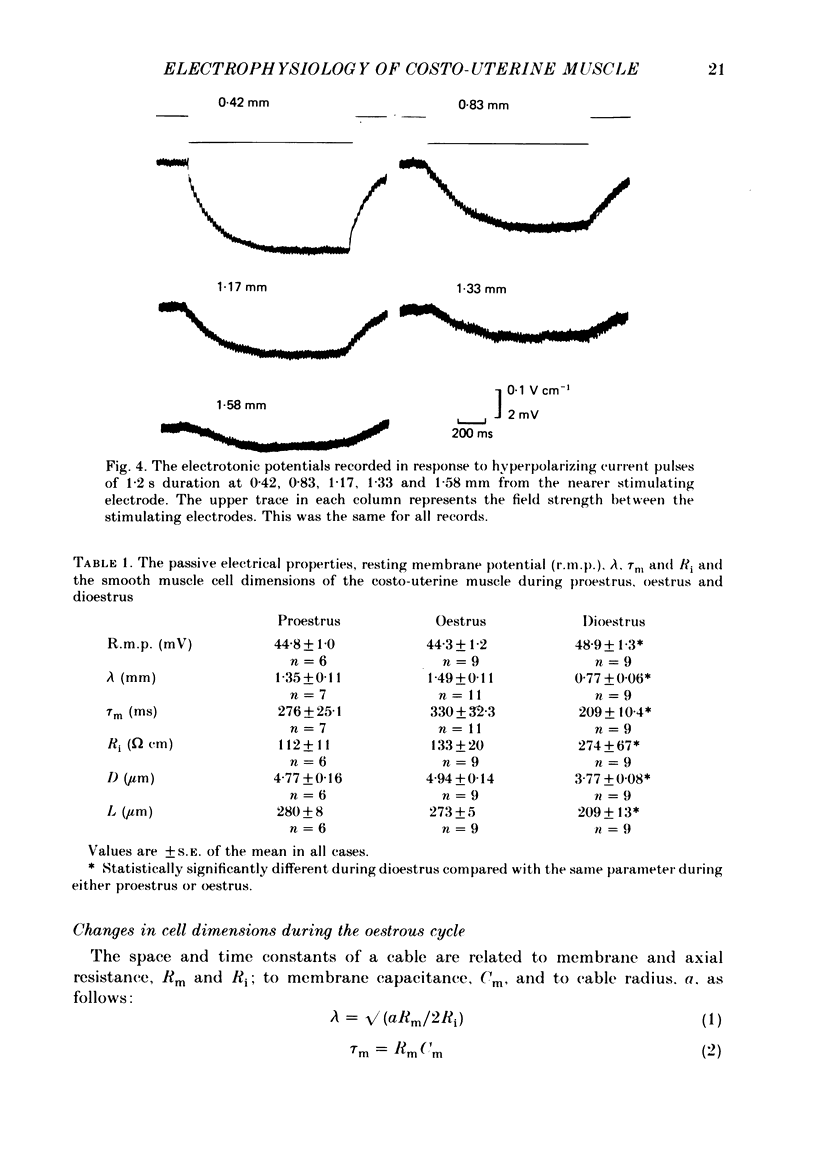
Abstract
The spontaneous electrical and mechanical activity of the costo-uterine muscle of the guinea-pig are described. The spontaneous electrical activity, recorded intracellularly, is similar to that observed previously in longitudinal myometrium of rat (Marshall, 1959) and ionic substitution suggests that, though calcium may be the predominant ion carrying the current during the upstroke of the action potential, some influence of sodium cannot be ruled out. During dioestrus, when circulating progesterone levels are high, there is an increase in the resting membrane potential and a decrease in the frequency of electrical and mechanical activity. There is a two-fold decrease in the space constant (lambda) during dioestrus. At this time the membrane time constant (tau m) is also decreased. The diameter and length of the smooth muscle cells are smaller during dioestrus. However, the differences in cell diameter do not explain all of the differences observed in lambda at this time and it is suggested that there may be an increase in the resistance to current flow between cells. It is concluded that high circulating progesterone may bring about quiescence of target smooth muscle in two ways: by stabilizing the cell membrane and by restricting the spread of activity.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y. Effects of changing the ionic environment on passive and active membrane properties of pregnant rat uterus. J Physiol. 1971 Apr;214(1):173–190. doi: 10.1113/jphysiol.1971.sp009426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODY S., WESTMAN A. Ovarian hormones and uterine growth. Effects of oestradiol and progesterone on cell growth and cell division in the rabbit uterus. Acta Obstet Gynecol Scand. 1960;39:557–565. doi: 10.3109/00016346009155734. [DOI] [PubMed] [Google Scholar]
- Bennett M. R. The effect of cations on the electrical properties of the smooth muscle cells of the guinea-pig vas deferens. J Physiol. 1967 Jun;190(3):465–479. doi: 10.1113/jphysiol.1967.sp008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatchley F. R., Donovan B. T., Ter Haar M. B. Plasma progesterone and gonadotrophin levels during the estrous cycle of the guinea pig. Biol Reprod. 1976 Aug;15(1):29–38. doi: 10.1095/biolreprod15.1.29. [DOI] [PubMed] [Google Scholar]
- Bo W. J., Odor D. L., Rothrock M. L. Ultrastructure of uterine smooth muscle following progesterone or progesterone-estrogen treatment. Anat Rec. 1969 Jan;163(1):121–131. doi: 10.1002/ar.1091630114. [DOI] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. The passive membrane properties and excitatory junction potentials of the guinea pig deferens. J Physiol. 1980 Mar;300:303–316. doi: 10.1113/jphysiol.1980.sp013163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Casteels R., Kuriyama H. Membrane potential and ion content in cat and guinea-pig myometrium and the response to adrenaline and noradrenaline. Br J Pharmacol. 1968 Oct;34(2):388–407. doi: 10.1111/j.1476-5381.1968.tb07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croix D., Franchimont P. Changes in the serum levels of the gonadotrophins progesterone and estradiol during the estrous cycle of the guinea pig. Neuroendocrinology. 1975;19(1):1–11. doi: 10.1159/000122420. [DOI] [PubMed] [Google Scholar]
- Gabella G. The costo-uterine muscle of the guinea-pig: a smooth muscle attaching the uterus to the last rib. Anat Embryol (Berl) 1976 Dec 22;150(1):35–43. doi: 10.1007/BF00346284. [DOI] [PubMed] [Google Scholar]
- Hernandez-Nicaise M. L., Mackie G. O., Meech R. W. Giant smooth muscle cells of Beroë. Ultrastructure, innervation, and electrical properties. J Gen Physiol. 1980 Jan;75(1):79–105. doi: 10.1085/jgp.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S., Bortoff A. Tissue resistance of the progesterone-dominated rabbit myometrium. Am J Physiol. 1970 Dec;219(6):1763–1767. doi: 10.1152/ajplegacy.1970.219.6.1763. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Changes in electrical properties of rat myometrium during gestation and following hormonal treatments. J Physiol. 1976 Sep;260(2):315–333. doi: 10.1113/jphysiol.1976.sp011517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngu M. C., Taylor G. S. Electrophysiological studies of the effects of sympathetic nerve stimulation and hormones on uterine motility. Comp Biochem Physiol A Comp Physiol. 1973 Jan 1;44(1):63–74. doi: 10.1016/0300-9629(73)90370-8. [DOI] [PubMed] [Google Scholar]
- Osa T. Effect of removing the external sodium on the electrical and mechanical activities of the pregnant mouse myometrium. Jpn J Physiol. 1971 Dec;21(6):607–625. doi: 10.2170/jjphysiol.21.607. [DOI] [PubMed] [Google Scholar]
- Parkington H. C., Lipton A. Sustained effects on synthetic ovarian steroids of rat myometrial contractility. J Endocrinol. 1976 Aug;70(2):223–227. doi: 10.1677/joe.0.0700223. [DOI] [PubMed] [Google Scholar]
- Sinback C. N., Shain W. Electrophysiological properties of human oviduct smooth muscle cells in dissociated cell culture. J Cell Physiol. 1979 Feb;98(2):377–393. doi: 10.1002/jcp.1040980214. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrophysiology of mammalian smooth muscle. Prog Biophys Mol Biol. 1975;30(2-3):185–203. doi: 10.1016/0079-6107(76)90009-2. [DOI] [PubMed] [Google Scholar]
- Tomita T. The longitudinal tissue impedance of the smooth muscle of guinea-pig taenia coli. J Physiol. 1969 Mar;201(1):145–159. doi: 10.1113/jphysiol.1969.sp008748. [DOI] [PMC free article] [PubMed] [Google Scholar]


