Abstract
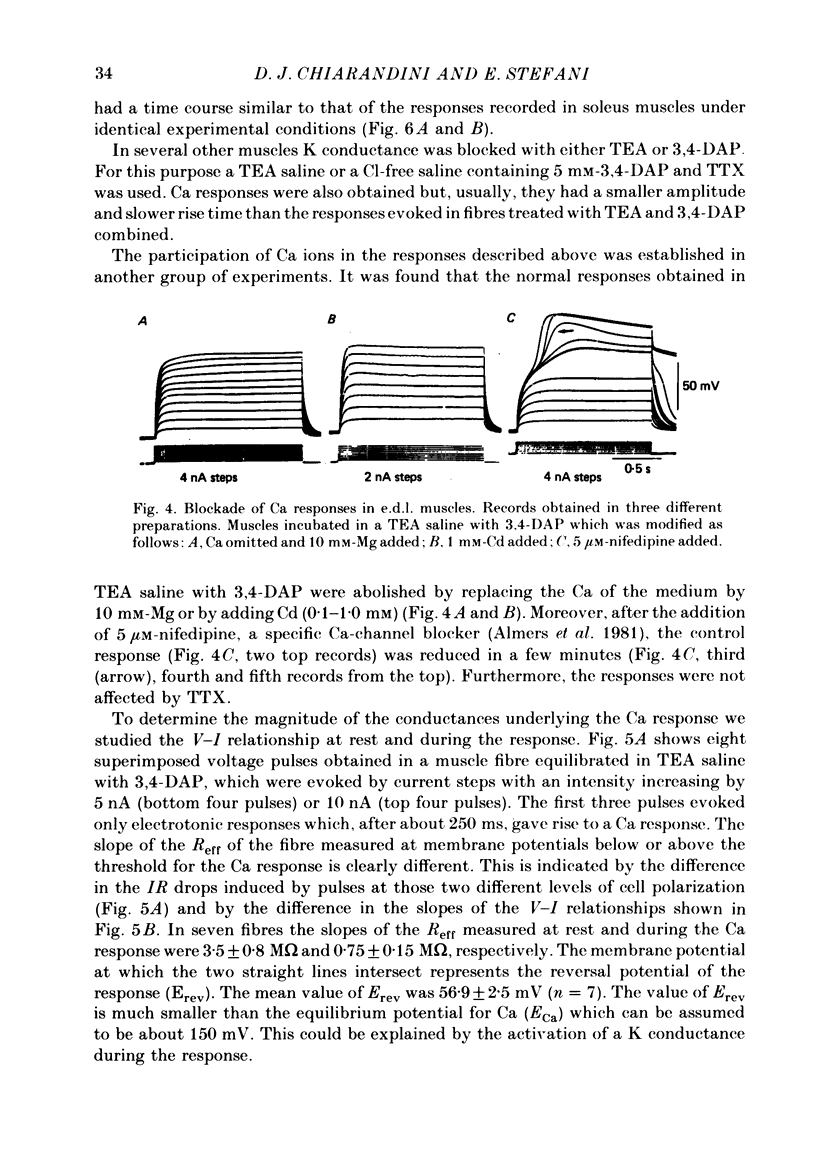
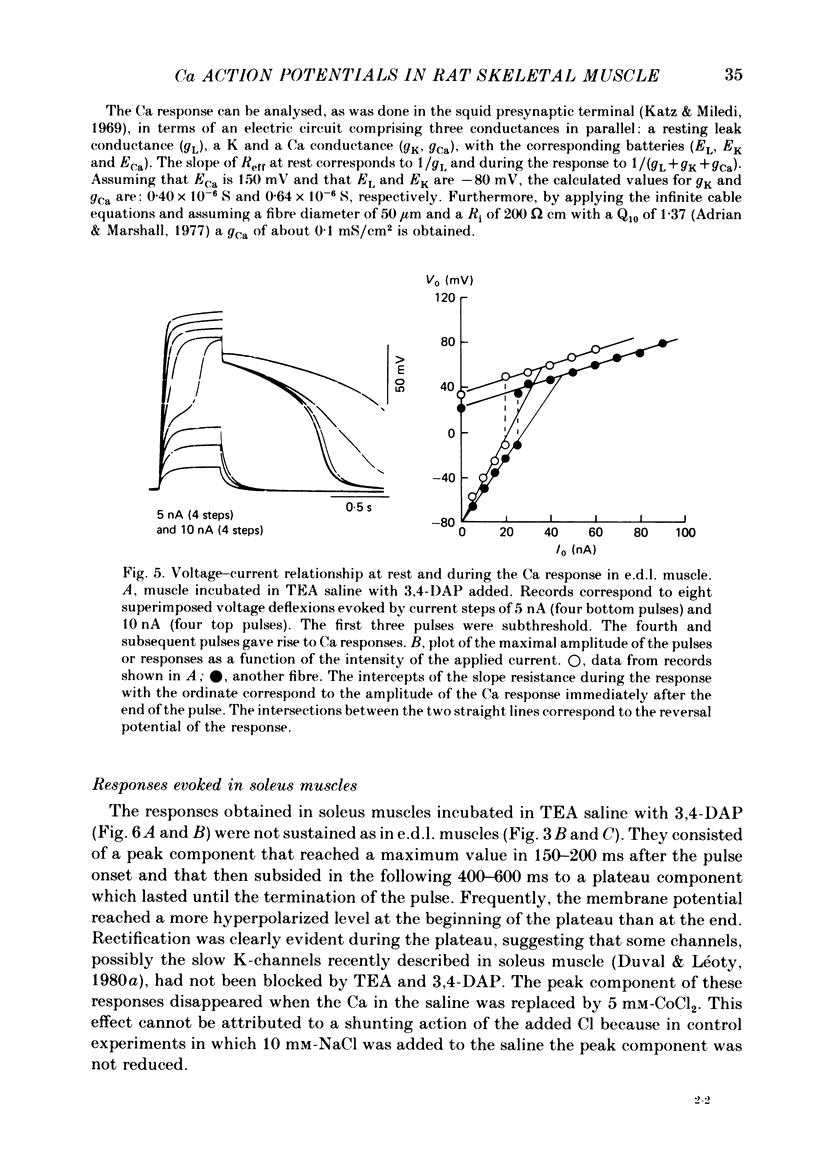
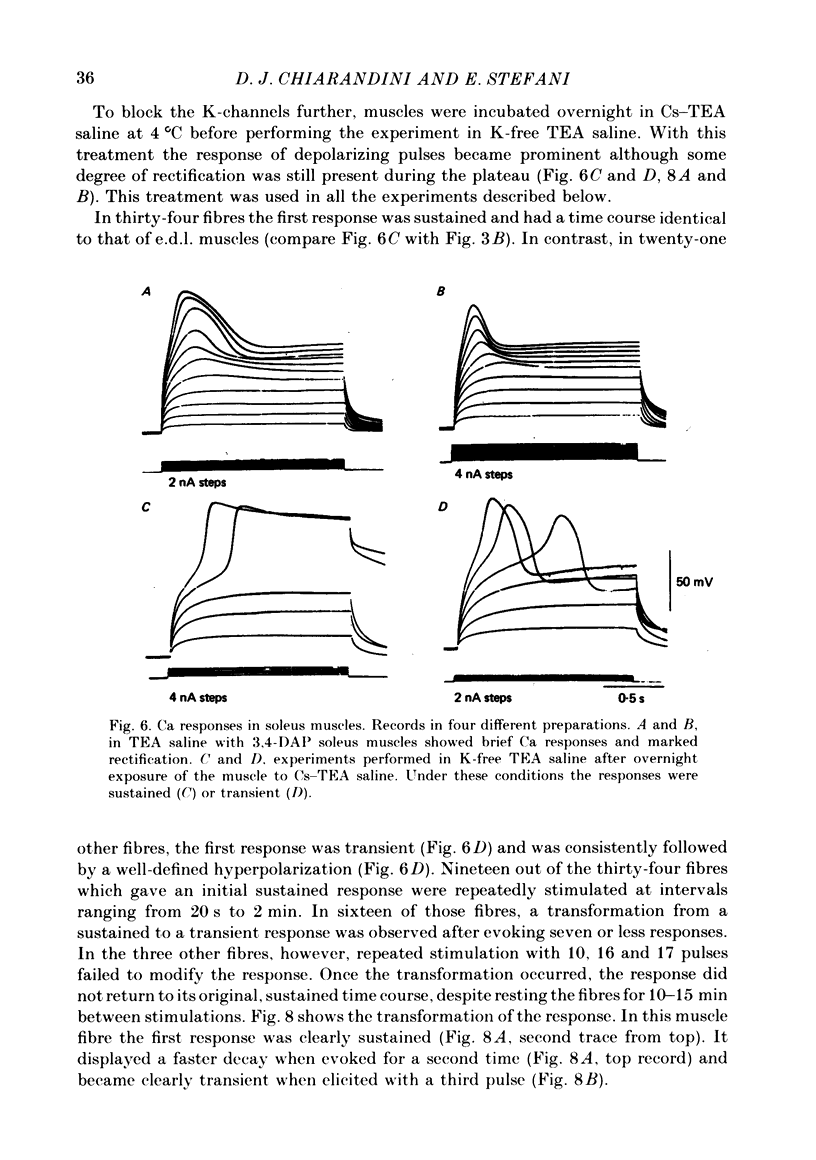
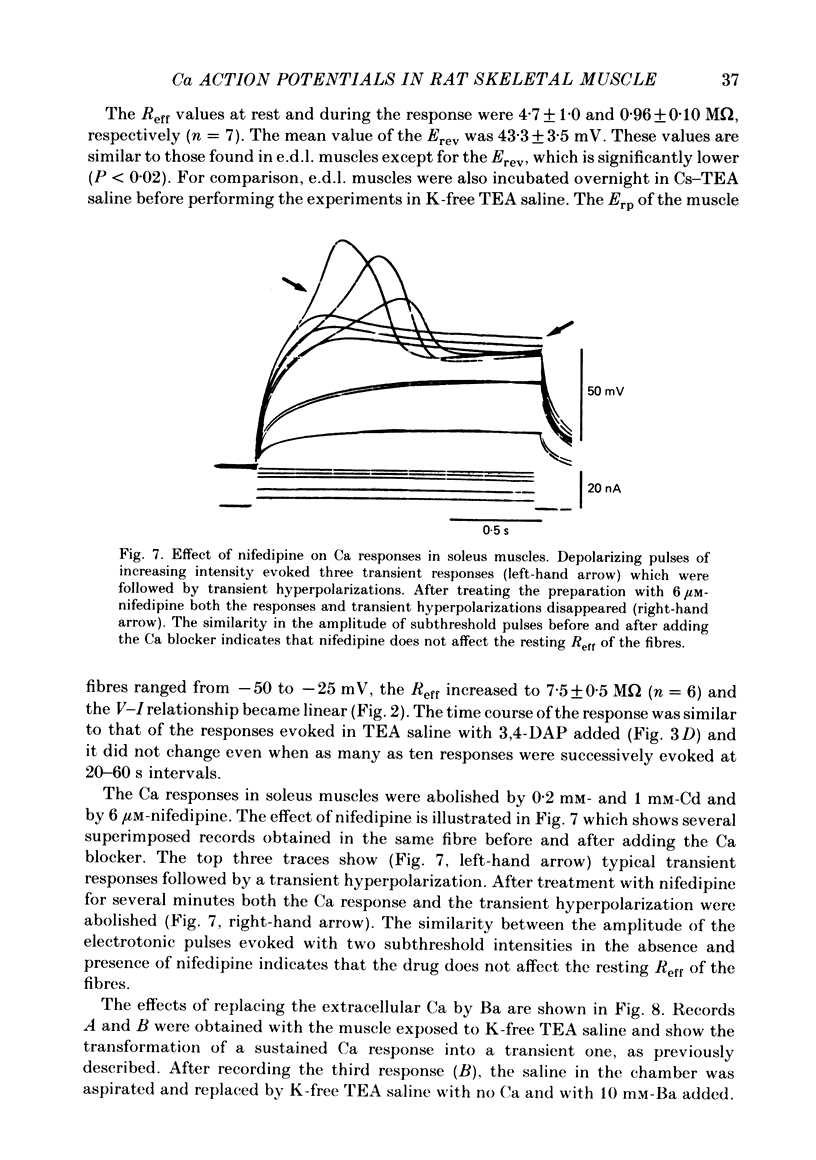
Slow action potentials were evoked in twitch fibres of rat extensor digitorum longus (e.d.l.) and soleus muscles after drastically reducing the Cl and K conductances of the muscle fibres. Cl conductance was eliminated by exposing the muscles to a Cl-free saline in which methanesulphonate replaced Cl. K conductance was reduced by adding tetraethylammonium (TEA) and 3,4-diaminopyridine (3,4-DAP) to the Cl-free saline or by overnight incubation of the muscles in a saline containing Cs and TEA. The delayed rectifier was markedly blocked by TEA and 3,4-DAP. In contrast, the inward rectifier was blocked only by TEA. Depolarization with pulses of increasing amplitude triggered slow responses which had a threshold of -30 to -10 mV and a peak amplitude of 50-60 mV. In e.d.l. muscles the time course of the response was sustained for the duration of the pulses and was not affected by repeated stimulation. In soleus muscles the first evoked response was sustained in about 60% of the fibres and transient in the rest. Transient responses reached a peak amplitude and were followed by a hyperpolarization. Repeated stimulation irreversibly transformed the sustained responses of soleus fibres into transient ones. The responses were blocked when the Ca in saline was replaced by Mg (10 mM) or Co (5 mM) or by the addition of Cd (0.1-1.0 mM) or nifedipine (5-6 microM). Tetrodotoxin did not affect the responses. These results strongly suggest that Ca is the main carrier of current during the response. Nifedipine blocked both the Ca response and the subsequent hyperpolarization, suggesting that the latter is due to the activation of a Ca-dependent K conductance.
Full text
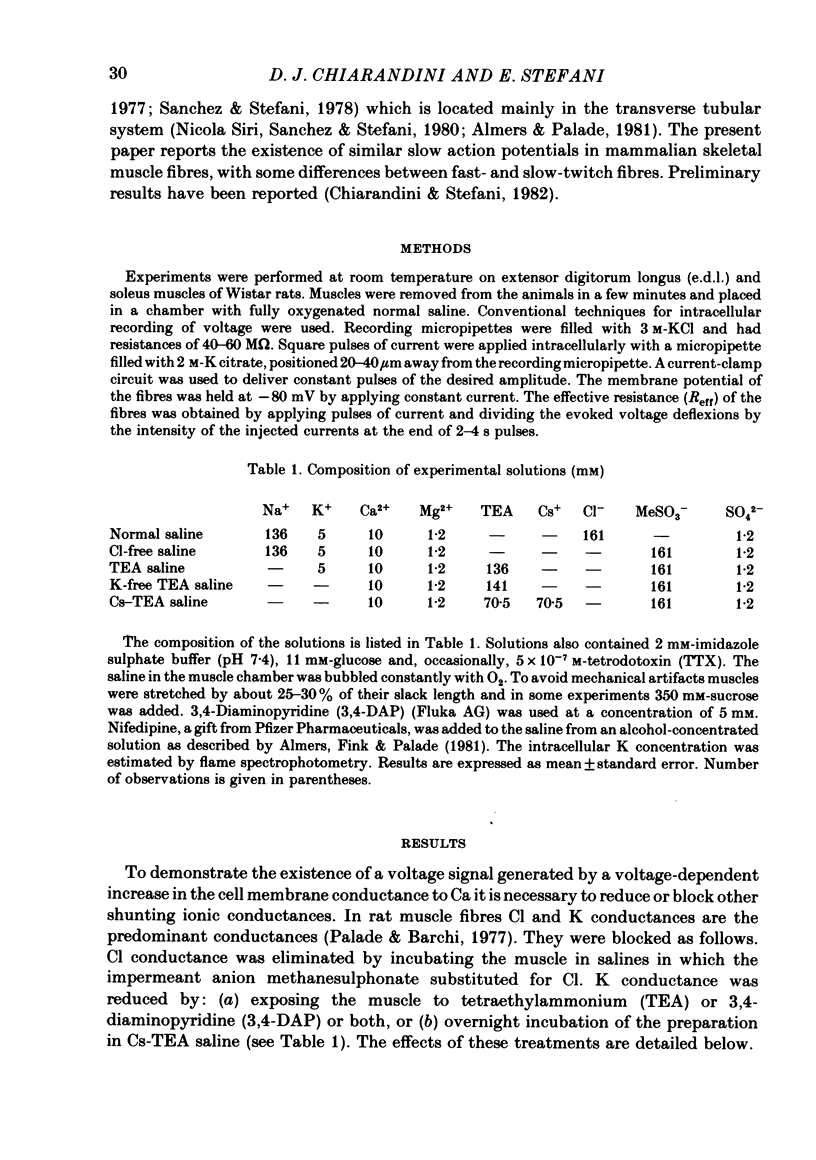
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Marshall M. W. Sodium currents in mammalian muscle. J Physiol. 1977 Jun;268(1):223–250. doi: 10.1113/jphysiol.1977.sp011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Fink R., Palade P. T. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981 Mar;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Palade P. T. Slow calcium and potassium currents across frog muscle membrane: measurements with a vaseline-gap technique. J Physiol. 1981 Mar;312:159–176. doi: 10.1113/jphysiol.1981.sp013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Leefmans F. J., Miledi R. Voltage sensitive calcium entry in frog motoneurones. J Physiol. 1980 Nov;308:241–257. doi: 10.1113/jphysiol.1980.sp013470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty G. N., Stefani E. Calcium dependent electrical activity in twitch muscle fibres of the frog. Proc R Soc Lond B Biol Sci. 1976 Aug 27;194(1114):141–150. doi: 10.1098/rspb.1976.0070. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Duval A., Léoty C. Comparison between the delayed outward current in slow and fast twitch skeletal muscle in the rat. J Physiol. 1980 Oct;307:43–57. doi: 10.1113/jphysiol.1980.sp013422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval A., Léoty C. Ionic currents in slow twitch skeletal muscle in the rat. J Physiol. 1980 Oct;307:23–41. doi: 10.1113/jphysiol.1980.sp013421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Changes in the intracellular concentration of free calcium ions in a pace-maker neurone, measured with the metallochromic indicator dye arsenazo III. J Physiol. 1978 Feb;275:357–376. doi: 10.1113/jphysiol.1978.sp012194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J Gen Physiol. 1977 Mar;69(3):325–342. doi: 10.1085/jgp.69.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Sanchez J. A., Stefani E. Inward calcium current in twitch muscle fibres of the frog. J Physiol. 1978 Oct;283:197–209. doi: 10.1113/jphysiol.1978.sp012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siri L. N., Sánchez J. A., Stefani E. Effect of glycerol treatment on the calcium current of frog skeletal muscle. J Physiol. 1980 Aug;305:87–96. doi: 10.1113/jphysiol.1980.sp013351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. A calcium dependent inward current in frog skeletal muscle fibres. Pflugers Arch. 1977 Apr 25;368(3):267–270. doi: 10.1007/BF00585206. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The differential effects of tetraethylammonium and zinc ions on the resting conductance of frog skeletal muscle. J Physiol. 1970 Jul;209(1):231–256. doi: 10.1113/jphysiol.1970.sp009164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERMAN R., GRUNDFEST H. Graded and all-or-none electrogenesis in arthropod muscle. II. The effects of alkali-earth and onium ions on lobster muscle fibers. J Gen Physiol. 1961 May;44:997–1027. doi: 10.1085/jgp.44.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]


