Abstract
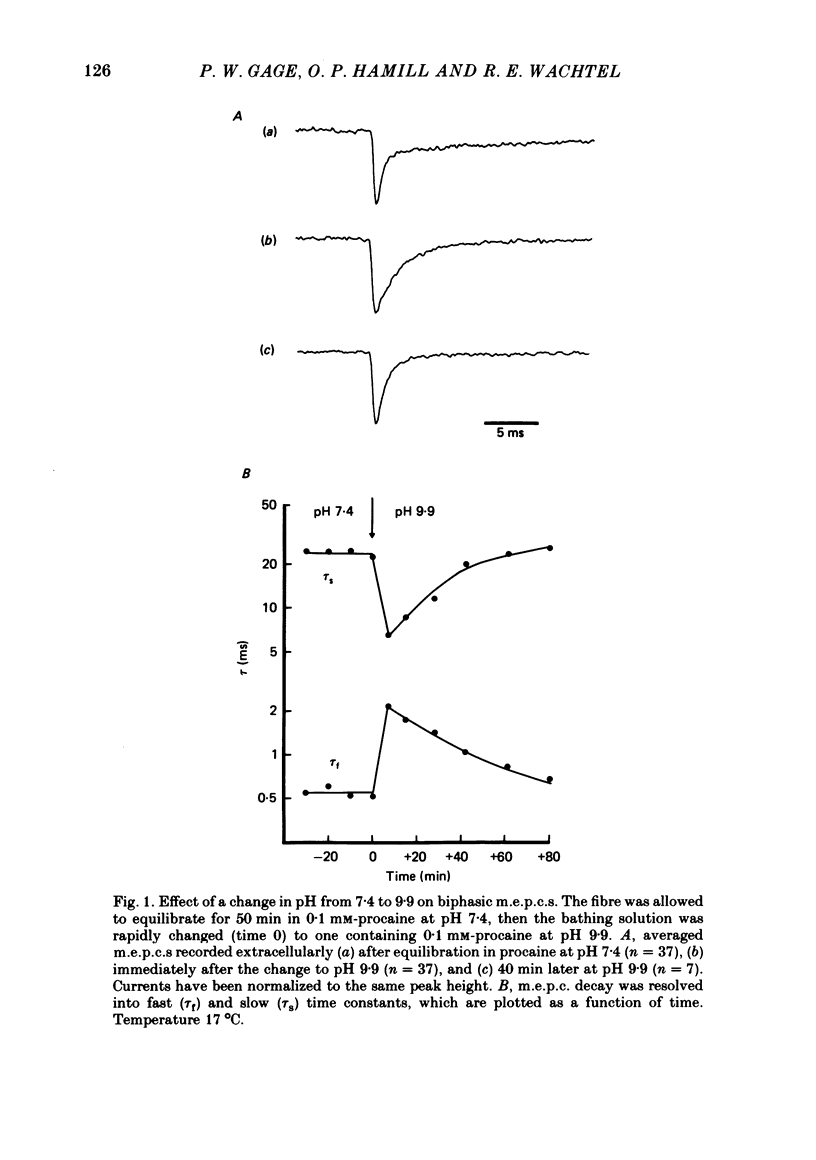
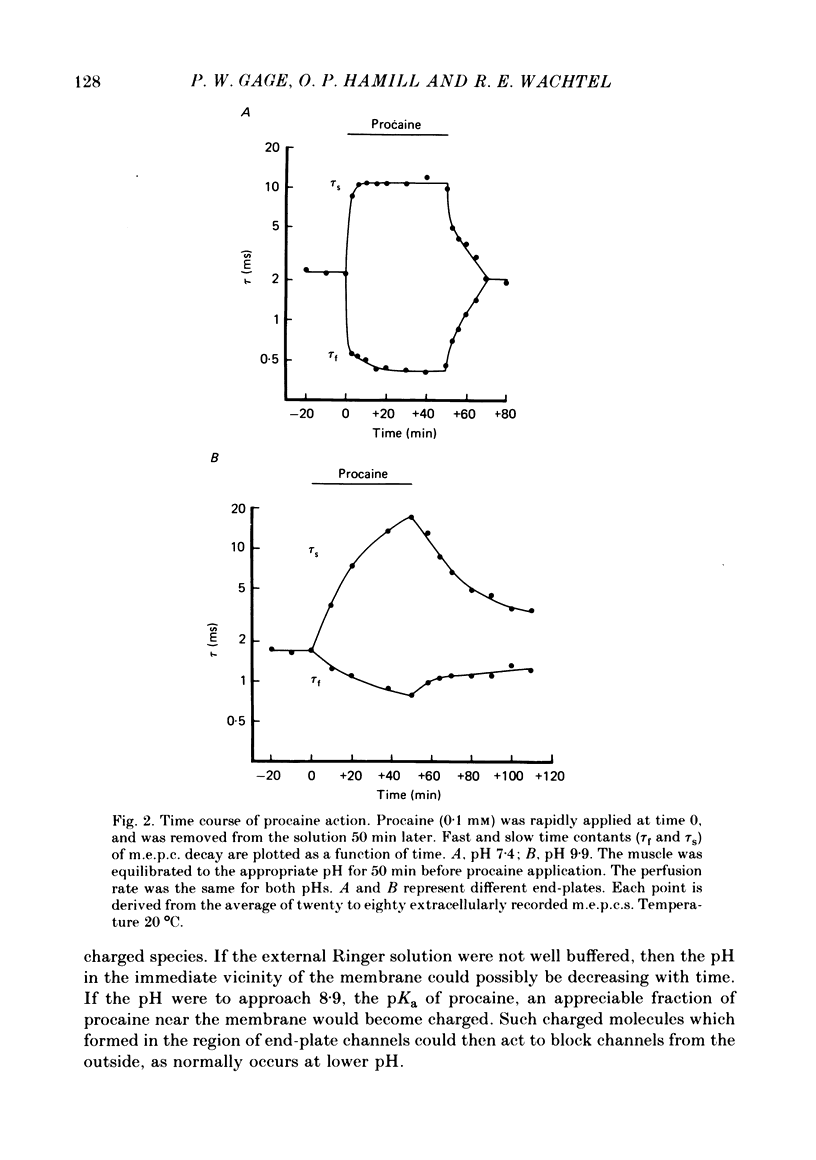
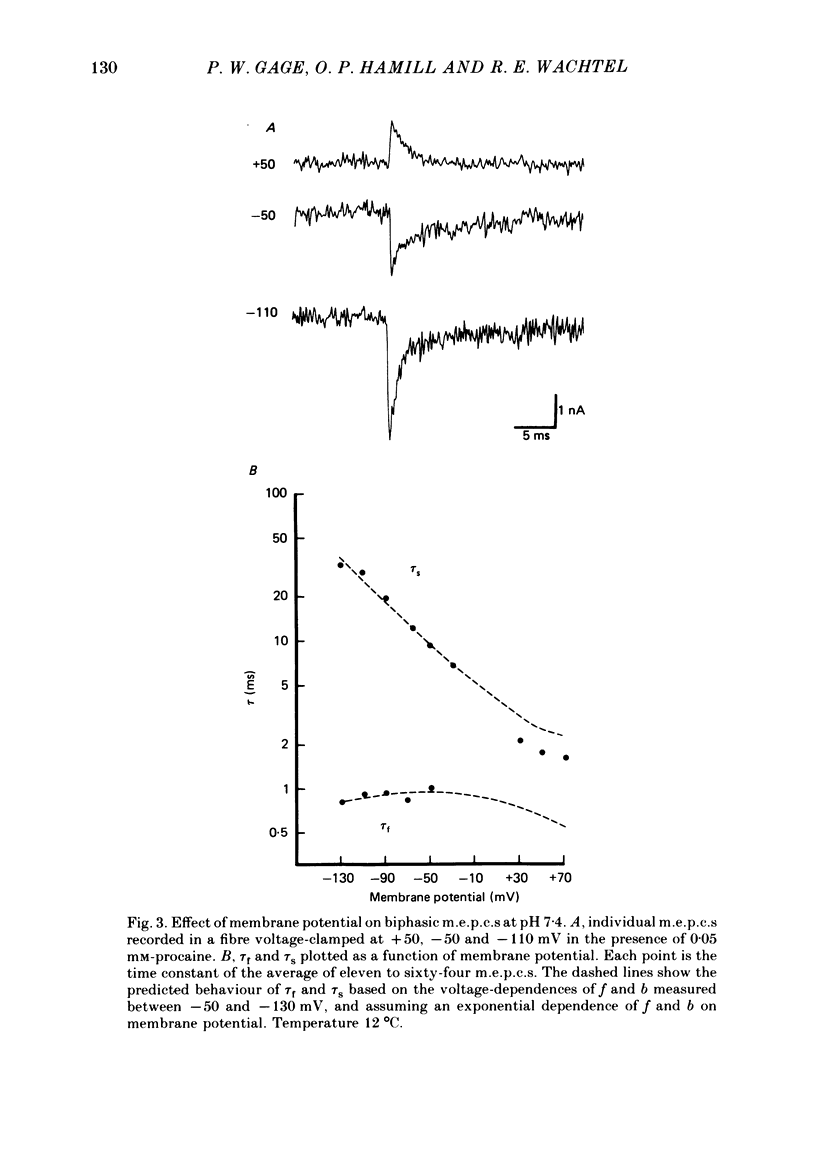
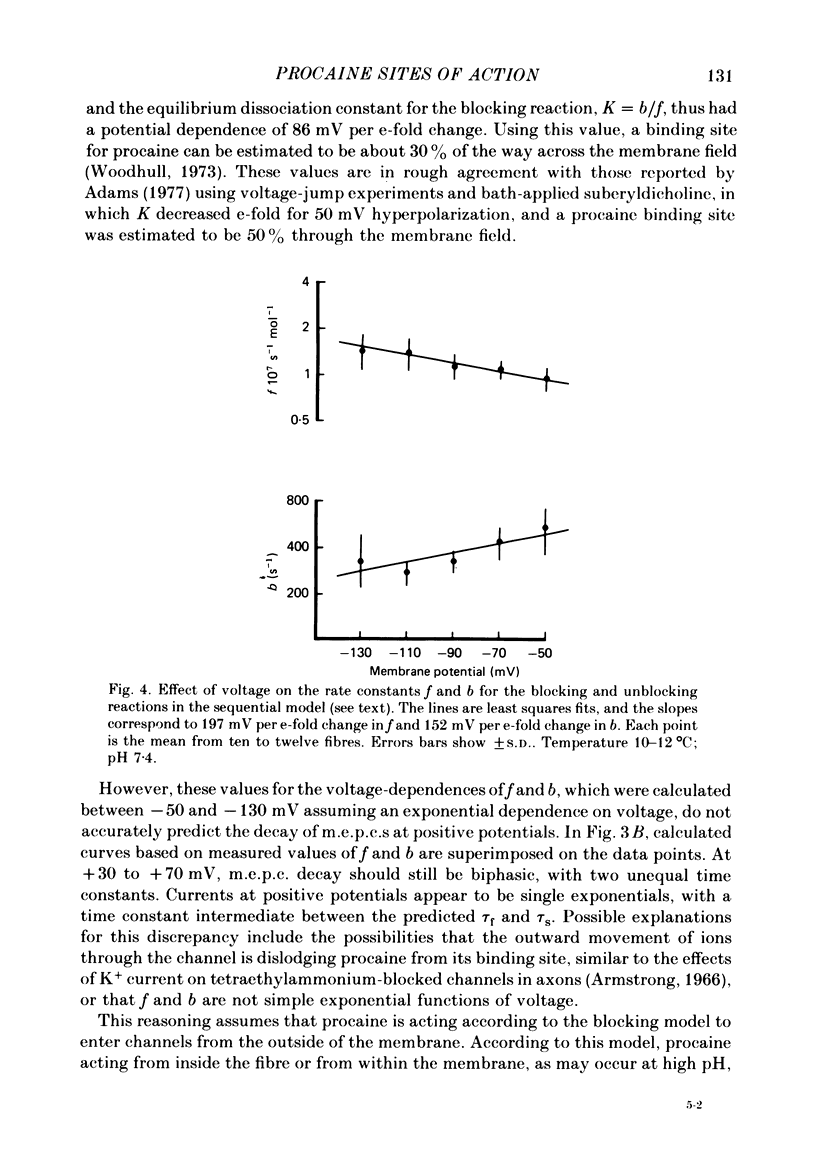
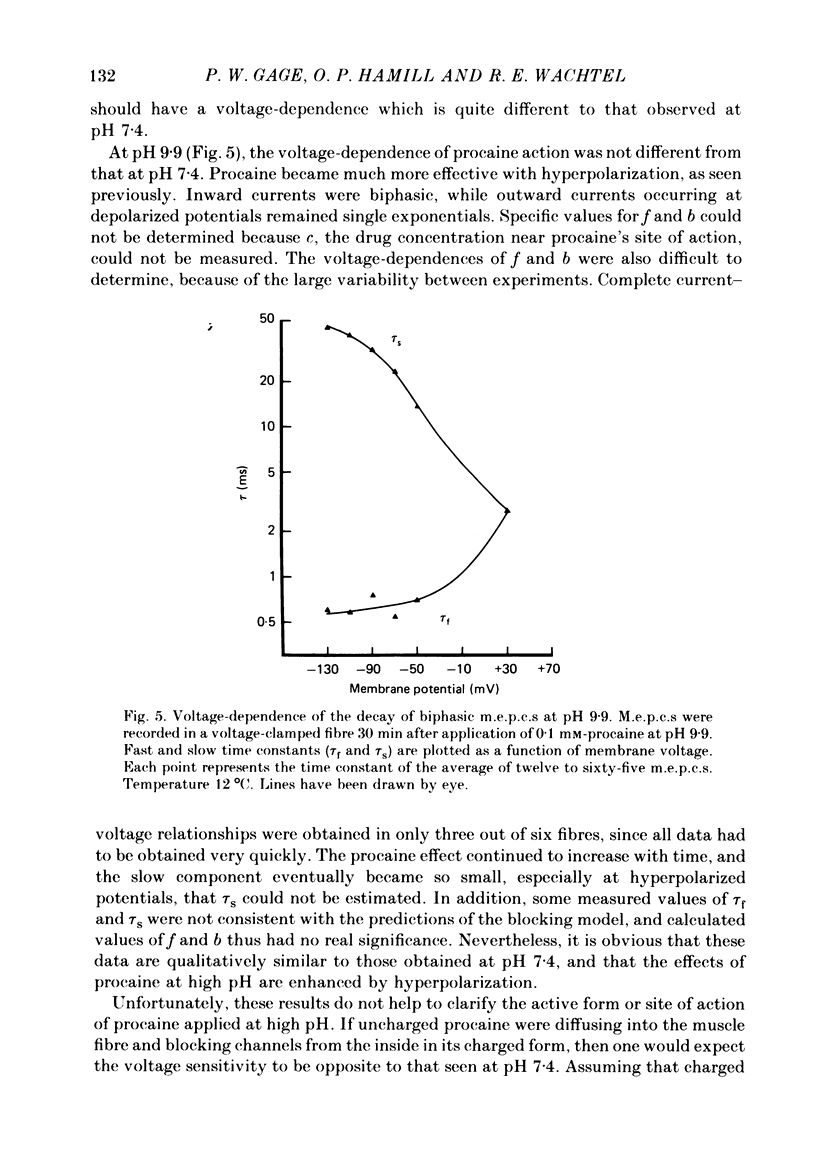
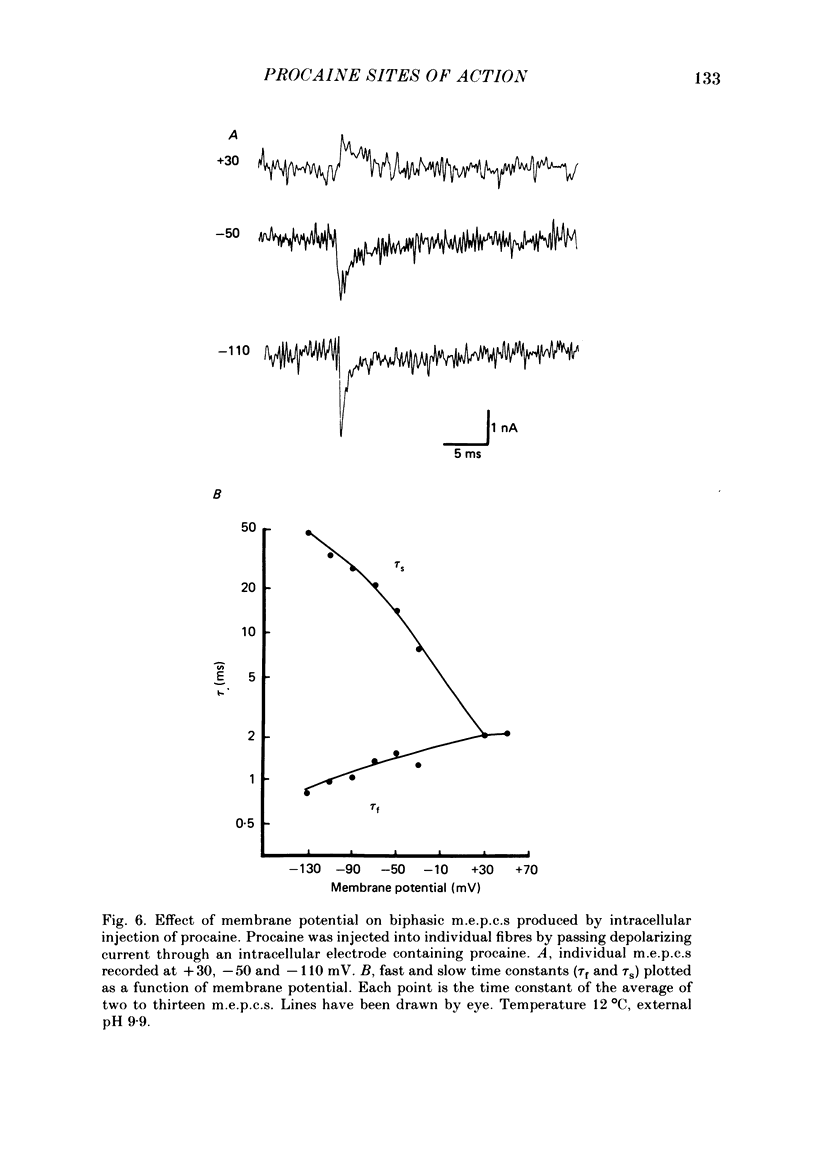
The effects of procaine at pH 7.4 and 9.9 were studied by examining the decay phase of spontaneous miniature end-plate currents (m.e.p.c.s) recorded from toad sartorius muscle fibres. Following exposure to procaine (0.05-0.1 mM) at pH 7.4, the decay of m.e.p.c.s rapidly became biphasic, and could be described as the sum of two exponential components. When the same concentrations of procaine were applied at pH 9.9, the development of biphasic m.e.p.c.s took much longer. Reversal of the effect upon washing out the procaine was much slower at pH 9.9 than at pH 7.4. A rapid change in pH from 7.4 to 9.9 during exposure to a constant concentration of procaine quickly reduced the effect of procaine on m.e.p.c.s. The effect gradually returned after prolonged exposure to procaine at pH 9.9. These results suggest that procaine applied at high pH, where it is predominantly in uncharged form, may be diffusing to a site of action which is not directly accessible from the external surface of the membrane. The voltage-dependence of procaine action was similar whether it was applied at pH 7.4 or 9.9. Intracellular injection of procaine rapidly produced biphasic m.e.p.c.s, whether the extracellular pH was 7.4 or 9.9. The effect of membrane potential on these m.e.p.c.s was similar to that seen for biphasic m.e.p.c.s produced by extracellular application of procaine. The results indicate that procaine can affect end-plate channels when applied to either surface of the muscle membrane, and that the voltage-dependence of procaine action does not arise from the influence of membrane field on the movement of charged procaine molecules into open channels.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. Decamethonium both opens and blocks endplate channels. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M., Albuquerque E. X., Lebeda F. J. Kinetic analysis of end plate currents altered by atropine and scopolamine. Mol Pharmacol. 1978 May;14(3):514–529. [PubMed] [Google Scholar]
- Adler M., Oliveira A. C., Eldefrawi M. E., Eldefrawi A. T., Albuquerque E. X. Tetraethylammonium: voltage-dependent action on endplate conductance and inhibition of ligand binding to postsynaptic proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):531–535. doi: 10.1073/pnas.76.1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo L. G., Pazhenchevsky B., Daly J. W., Albuquerque E. X. The ionic channel of the acetylcholine receptor. Regulation by sites outside and inside the cell membrane which are sensitive to quaternary ligands. Mol Pharmacol. 1981 Sep;20(2):345–355. [PubMed] [Google Scholar]
- Albuquerque E. X., Kuba K., Daly J. Effect of histrionicotoxin on the ionic conductance modulator of the cholinergic receptor: a quantitative analysis of the end-plate current. J Pharmacol Exp Ther. 1974 May;189(2):513–524. [PubMed] [Google Scholar]
- Armstrong C. M. Time course of TEA(+)-induced anomalous rectification in squid giant axons. J Gen Physiol. 1966 Nov;50(2):491–503. doi: 10.1085/jgp.50.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G. A voltage-clamp study of the effect of two lidocaine derivatives on the time course of end-plate currents. J Physiol. 1976 Jun;258(2):279–300. doi: 10.1113/jphysiol.1976.sp011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sheridan R. E. The modes of action of gallamine. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):181–203. doi: 10.1098/rspb.1981.0002. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Narahashi T. Effects of procaine on ionic conductances of end-plate membranes. J Pharmacol Exp Ther. 1971 Feb;176(2):423–433. [PubMed] [Google Scholar]
- Gage P. W., Armstrong C. M. Miniature end-plate currents in voltage-clamped muscle fibre. Nature. 1968 Apr 27;218(5139):363–365. doi: 10.1038/218363b0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Sah P. Postsynaptic effects of some central stimulants at the neuromuscular junction. Br J Pharmacol. 1982 Mar;75(3):493–502. doi: 10.1111/j.1476-5381.1982.tb09166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The pH-dependent rate of action of local anesthetics on the node of Ranvier. J Gen Physiol. 1977 Apr;69(4):475–496. doi: 10.1085/jgp.69.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Brodwick M. S., Dickey W. D. Asymmetry of the acetylcholine channel revealed by quaternary anesthetics. Science. 1980 Oct 10;210(4466):205–207. doi: 10.1126/science.6251552. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of procaine on the action of acetylcholine at the neuromuscular junction. J Physiol. 1975 Jul;249(2):269–284. doi: 10.1113/jphysiol.1975.sp011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas M. The effect of procaine on neuromuscular transmission. J Physiol. 1970 Aug;209(3):689–699. doi: 10.1113/jphysiol.1970.sp009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau E. M., Gavish B., Nachshen D. A., Lotan I. pH dependence of the acetylcholine receptor channel: a species variation. J Gen Physiol. 1981 Jun;77(6):647–666. doi: 10.1085/jgp.77.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno T. Analysis of sodium and potassium conductances in the procaine end-plate potential. J Physiol. 1966 Apr;183(3):592–606. doi: 10.1113/jphysiol.1966.sp007886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno T., Edwards C., Hashimura S. Difference in effects of end-plate potentials between procaine and lidocaine as revealed by voltage-clamp experiments. J Neurophysiol. 1971 Jan;34(1):32–46. doi: 10.1152/jn.1971.34.1.32. [DOI] [PubMed] [Google Scholar]
- Mallart A., Molgó J. The effects of pH and curare on the time course of end-plate currents at the neuromuscular junction of the frog. J Physiol. 1978 Mar;276:343–352. doi: 10.1113/jphysiol.1978.sp012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Blocking of acetylcholine-induced channels by extracellular or intracellular application of D600. Proc R Soc Lond B Biol Sci. 1980 Dec 31;211(1182):143–150. doi: 10.1098/rspb.1980.0162. [DOI] [PubMed] [Google Scholar]
- Milne R. J., Byrne J. H. Effects of hexamethonium and decamethonium on end-plate current parameters. Mol Pharmacol. 1981 Mar;19(2):276–281. [PubMed] [Google Scholar]
- Narahashi T., Frazier D. T., Moore J. W. Comparison of tertiary and quaternary amine local anesthetics in their ability to depress membrane ionic conductances. J Neurobiol. 1972;3(3):267–276. doi: 10.1002/neu.480030309. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Frazier D. T. Site of action and active form of procaine in squid giant axons. J Pharmacol Exp Ther. 1975 Sep;194(3):506–513. [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D. C., Siegelbaum S. A., Colquhoun D. Block of acetylcholine-activated ion channels by an uncharged local anaesthetic. Nature. 1981 Feb 12;289(5798):596–598. doi: 10.1038/289596a0. [DOI] [PubMed] [Google Scholar]
- Ohki S., Gravis C., Pant H. Permeability of axon membranes to local anesthetics. Biochim Biophys Acta. 1981 May 6;643(2):495–507. doi: 10.1016/0005-2736(81)90091-2. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Ruff R. L. The kinetics of local anesthetic blockade of end-plate channels. Biophys J. 1982 Mar;37(3):625–631. [PMC free article] [PubMed] [Google Scholar]
- Scuka M. The amplitude and the time course of the end-plate current at various pH levels in the frog sartorius muscle. J Physiol. 1975 Jul;249(2):183–195. doi: 10.1113/jphysiol.1975.sp011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. C., Mansour N. A., Eldefrawi A. T., Eldefrawi M. E., Albuquerque E. X. Mechanism of action of amantadine on neuromuscular transmission. Mol Pharmacol. 1978 Sep;14(5):787–803. [PubMed] [Google Scholar]


