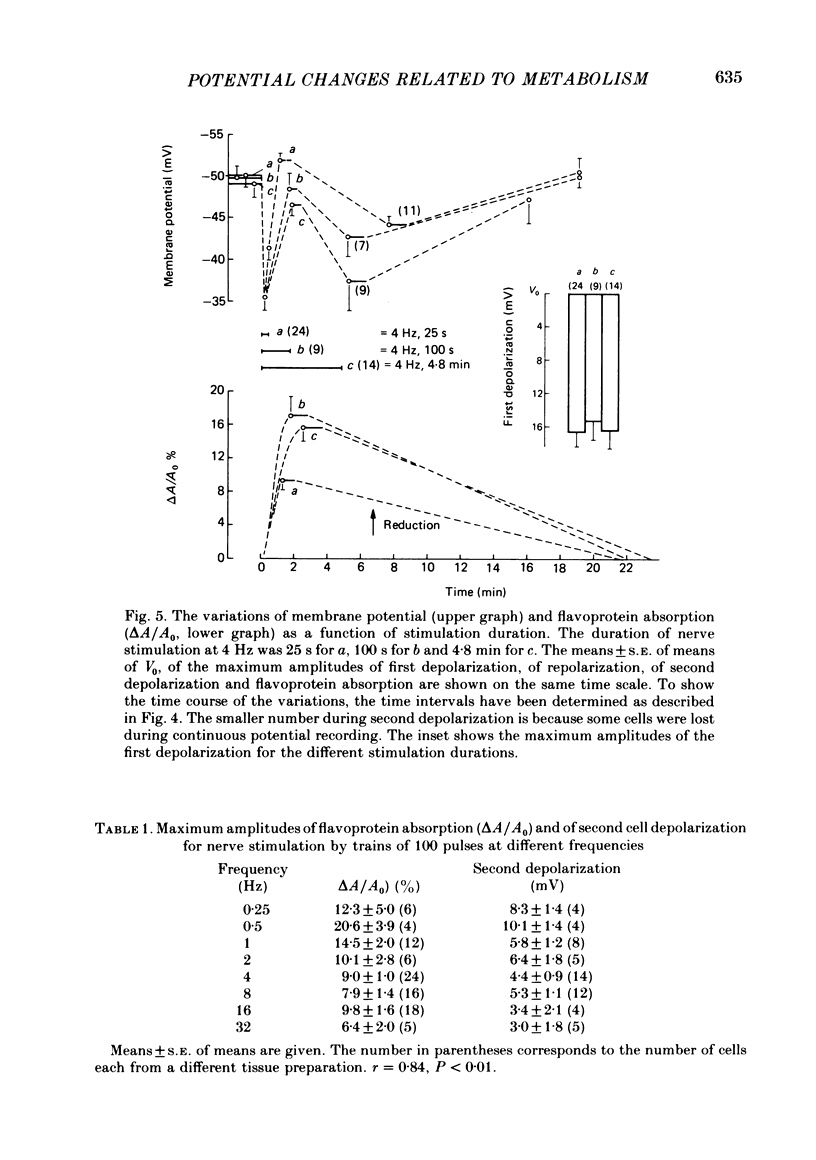
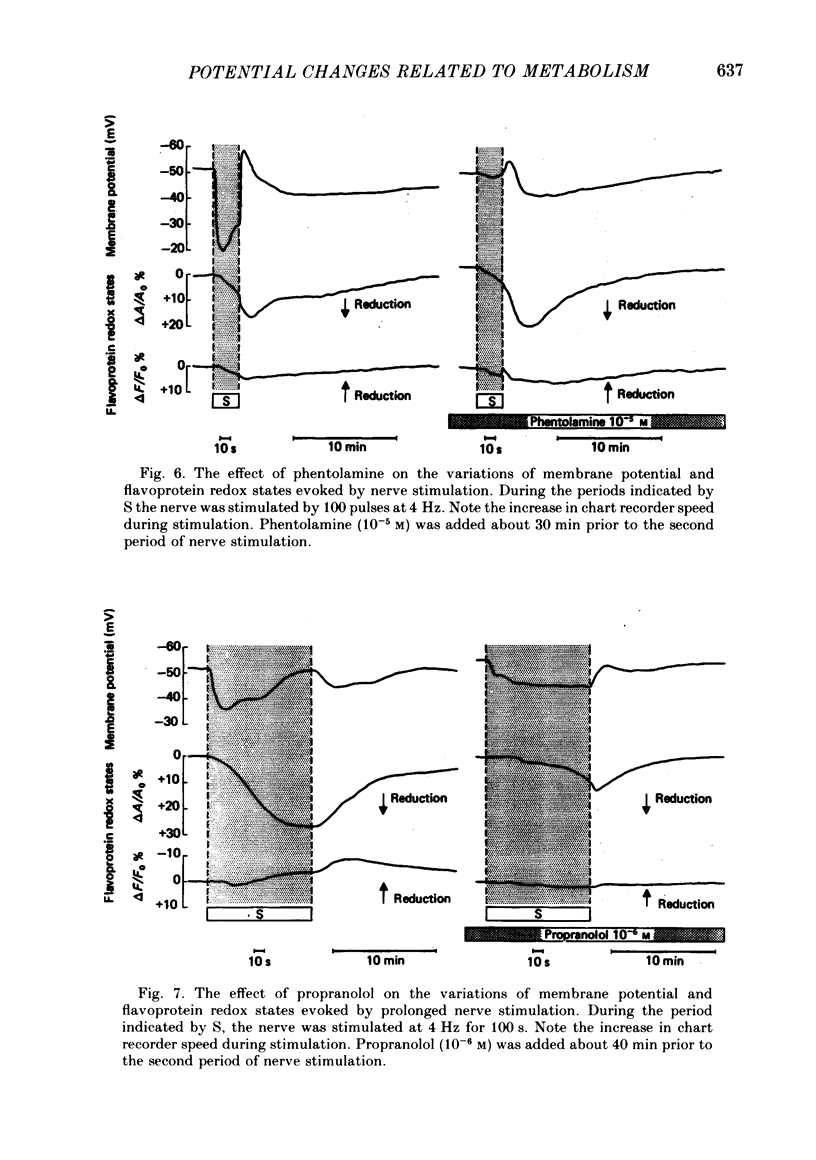
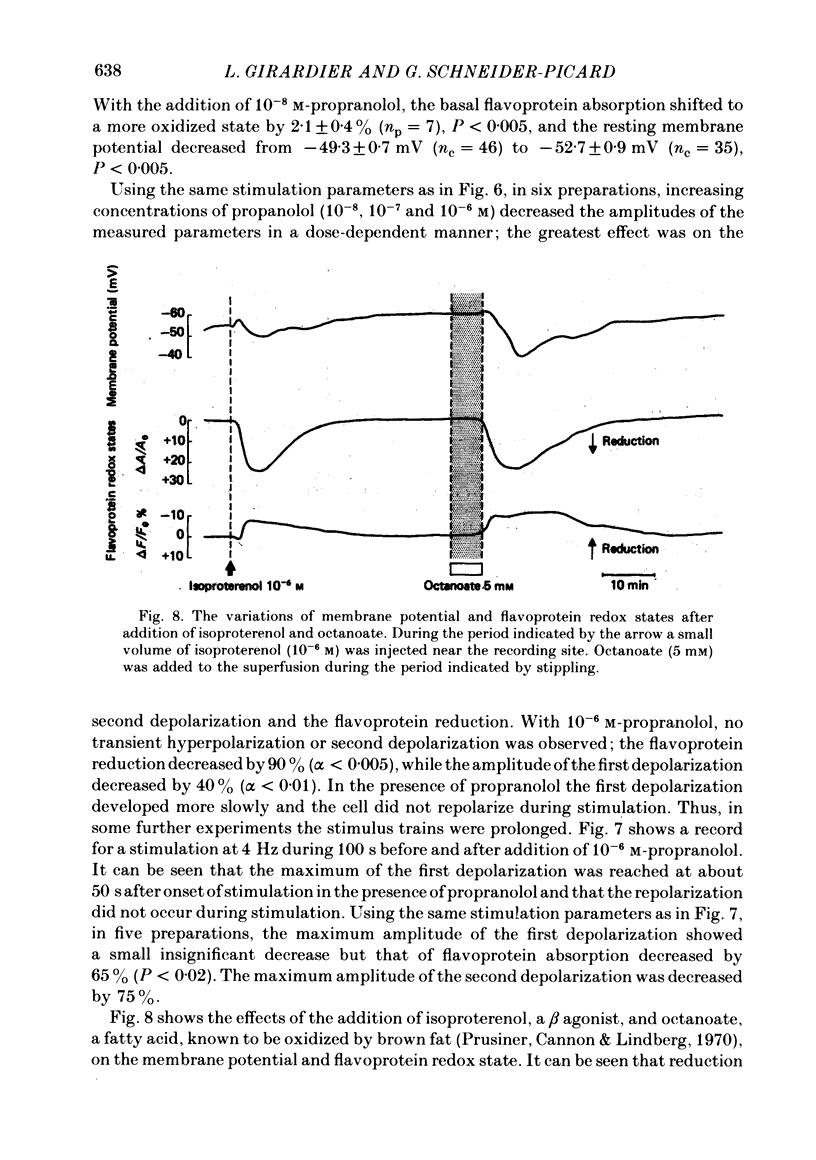
Abstract
Membrane potential and flavoprotein redox state have been measured simultaneously and continuously in brown adipose tissue in order to determine how nerve stimulation and adrenergic agonists control its metabolic activity. Both trains of nerve impulses and addition of noradrenaline evoke two temporally distinct cell depolarizations. The first rapid depolarization precedes the increase in flavoprotein reduction. With nerve stimulation, at the time of maximum flavoprotein reduction the cell has repolarized or hyperpolarized. The second slow depolarization follows flavoprotein reduction. Phentolamine, an alpha antagonist, selectively blocks the first depolarization, but not the flavoprotein reduction. However the time of maximum flavoprotein reduction is delayed. Propranolol, a beta antagonist, delays the first repolarization until the end of nerve stimulation and inhibits the transient hyperpolarization, second depolarization and flavoprotein reduction. Isoproterenol, a beta agonist, or the fatty acid octanoate produce only a transient hyperpolarization and subsequent slow depolarization following flavoprotein reduction. Thus brown adipose tissue contains both alpha- and beta-adrenergic receptors. Stimulation of alpha receptors produces an early membrane depolarization. Stimulation of beta receptors leads to an increase in metabolic activity which then appears to produce slow changes in membrane potential.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Exton J. H. Molecular mechanisms involved in alpha-adrenergic responses. Mol Cell Endocrinol. 1981 Sep;23(3):233–264. doi: 10.1016/0303-7207(81)90123-4. [DOI] [PubMed] [Google Scholar]
- Fain J. N. Dihydroergotamine, propranolol and the beta adrenergic receptors of fat cells. Fed Proc. 1970 Jul-Aug;29(4):1402–1407. [PubMed] [Google Scholar]
- Fink S. A., Williams J. A. Adrenergic receptors mediating depolarization in brown adipose tissue. Am J Physiol. 1976 Sep;231(3):700–706. doi: 10.1152/ajplegacy.1976.231.3.700. [DOI] [PubMed] [Google Scholar]
- Fromm M., Weskamp P., Hegel U. Versatile piezoelectric driver for cell puncture. Pflugers Arch. 1980 Mar;384(1):69–73. doi: 10.1007/BF00589517. [DOI] [PubMed] [Google Scholar]
- Girardier L., Seydoux J., Clausen T. Membrane potential of brown adipose tissue. A suggested mechanism for the regulation of thermogenesis. J Gen Physiol. 1968 Dec;52(6):925–940. doi: 10.1085/jgp.52.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardier L., Seydoux J. Le contrôle de la thermogenése du tissu adipeux brun. J Physiol (Paris) 1971 Mar-Apr;63(2):147–186. [PubMed] [Google Scholar]
- Horwitz B. A., Horowitz J. M., Jr, Smith R. E. Norepinephrine-induced depolarization of brown fat cells. Proc Natl Acad Sci U S A. 1969 Sep;64(1):113–120. doi: 10.1073/pnas.64.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Moskowitz J., Dempsey P., Brodie B. B. The effect of norepinephrine and insulin on brown fat cell membrane potentials. Life Sci I. 1970 Dec 1;9(23):1353–1361. doi: 10.1016/0024-3205(70)90043-3. [DOI] [PubMed] [Google Scholar]
- Reed N., Fain J. N. Stimulation of respiration in brown fat cells by epinephrine, dibutyryl-3',5'-adenosine monophosphate, and m-chloro(carbonyl cyanide)phenylhydrazone. J Biol Chem. 1968 Jun 10;243(11):2843–2848. [PubMed] [Google Scholar]
- Revel J. P., Yee A. G., Hudspeth A. J. Gap junctions between electrotonically coupled cells in tissue culture and in brown fat. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2924–2927. doi: 10.1073/pnas.68.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979 Sep 6;281(5726):31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Sada H., Ban T. Frequency-dependent block of nerve conduction by beta-adrenergic blocking agents. Arch Int Pharmacodyn Ther. 1981 Nov;254(1):134–144. [PubMed] [Google Scholar]
- Schneider-Picard G., Carpentier J. L., Orci L. Quantitative evaluation of gap junctions during development of the brown adipose tissue. J Lipid Res. 1980 Jul;21(5):600–607. [PubMed] [Google Scholar]
- Schneider-Picard G., Girardier L. Postnatal development of sympathetic innervation of rat brown adipose tissue reevaluated with a method allowing for monitoring flavoprotein redox state. J Physiol (Paris) 1982 Aug;78(2):151–157. [PubMed] [Google Scholar]
- Seydoux J., Constantinidis J., Tsacopoulos M., Girardier L. In vitro study of the control of the metabolic activity of brown adipose tissue by the sympathetic nervous system. J Physiol (Paris) 1977;73(7):985–976. [PubMed] [Google Scholar]
- Sheridan J. D. Electrical coupling between fat cells in newt fat body and mouse brown fat. J Cell Biol. 1971 Sep;50(3):795–803. doi: 10.1083/jcb.50.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., James W. P. Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse. Pflugers Arch. 1978 Feb 22;373(2):189–193. doi: 10.1007/BF00584859. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Matthews E. K. Membrane depolarization, cyclic AMP, and glycerol release by brown adipose tissue. Am J Physiol. 1974 Oct;227(4):987–992. doi: 10.1152/ajplegacy.1974.227.4.987. [DOI] [PubMed] [Google Scholar]


