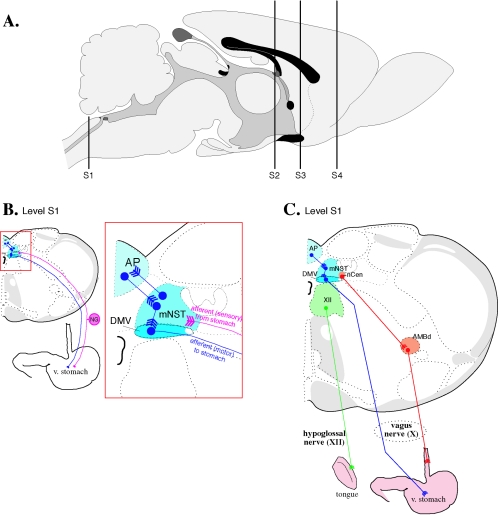
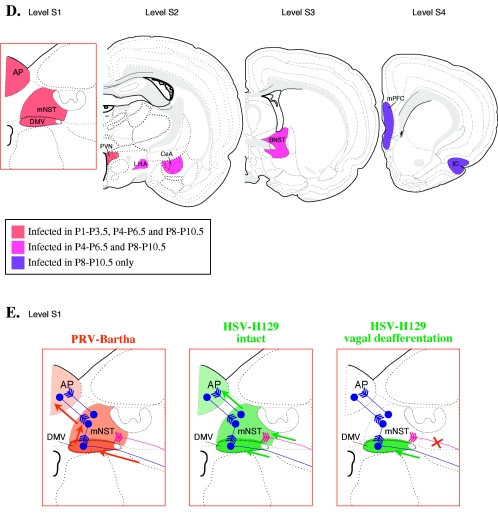
FIG. 4.
Stomach injection model. A. Sagittal view of the rat brain. S1, S2, and S3 refer to the levels of coronal sections depicted in panels B, C, D, and E. B. Innervation of smooth muscle of the ventral stomach. The area boxed in red is magnified to the right. Motor neurons from the dorsal motor nucleus of the vagus send projections through the vagus nerve to the ventral wall of the stomach. Sensory innervation of the ventral stomach through the left nodose ganglion is shown in pink. Neurons in the dorsal motor nucleus of the vagus exhibit anti-PRV immune reactivity 30 h after stomach injection of wild-type PRV-Becker (64, 338). PRV travels retrogradely to second-order neurons in the medial nucleus of the solitary tract between 50 and 60 h postinjection and to third-order neurons in the area postrema between 60 and 70 h postinjection. Labeling of neurons within the left nodose ganglia can be observed by 45 and 50 h postinjection. C. No cross talk with tongue or esophageal innervation after stomach injection. Injection of PRV-Becker into the ventrolateral musculature of the tongue (pathway shown in green) results in a very different pattern of infection from injection into the stomach (shown in blue) (64). After transport through the hypoglossal nerve, PRV immune reactivity can be seen in the hypoglossal nucleus (XII) 30 h postinjection. By about 52 h postinjection, PRV infection can be observed in second-order neurons in the spinal trigeminal nucleus (pars oralis and pars interpolaris) and the ventrolateral brainstem tegumentum and monoaminergic cell groups (not shown). Injection into the smooth muscle of the esophagus (shown in orange) produced labeling in the dorsal nucleus ambiguus. By 48 hours postinfection, labeling was detected in small bipolar neurons of the nucleus centralis of the medial NTS. The segregation of labeled structures following injection of stomach and esophagus is significant because the axons of these efferent circuits travel together in the vagus nerve, yet PRV infection is absent from the nucleus ambiguous following stomach injection and absent from the dorsal motor nucleus of the vagus followingesophageal injection. D. PRV requires an intact circuit for spread in the nervous system. In addition to surgical severance of the left vagus nerve which eliminates PRV transport to the left dorsal motor nucleus of the vagus, further proof that PRV neuronal spread requires intact, synaptically connected neurons is provided by tracing studies that span progressing developmental stages (339). PRV-Bartha immune reactivity in the central nervous system was examined 2.5 days after injection into the stomachs of newborn rats. Rats injected on postnatal day 1 (P1) exhibited PRV immune reactivity in the dorsal motor nucleus of the vagus, medial nucleus of the solitary tract, area postrema, and paraventricular nucleus of the hypothalamus by 2.5 days postinjection. No animals in the P1 group exhibited anti-PRV labeling in the central nucleus of the amygdala, lateral hypothalamic area, bed nucleus of the stria terminalis, insular cortex, or medial prefrontal cortex with the exception of one rat with six labeled neurons in the central nucleus of the amygdala. Rats injected at later developmental stages exhibit progressively more viral penetrance into the central nervous system; 2.5 days postinjection, P4 rats exhibit labeling of all structures observed in the P1 group plus extensive labeling in the central nucleus of the amygdala, lateral hypothalamic area, and bed nucleus of the stria terminalis. Only rats injected on P8 exhibit infection of neurons in the insular cortex and medial prefrontal cortex. E. Comparison of anterograde- and retrograde-defective alphaherpesviruses. In adult rats, stomach injection of PRV-Bartha results in retrograde-only transport of viral infection (pathway and PRV-immune reactive structures shown in orange) from the dorsal motor nucleus of the vagus to medial nucleus of the solitary tract to area postrema (340). Anti-PRV immunoreactivity 4 to 5 days postinjection does not change after elimination of anterograde transport by surgically severing the axons of pseudounipolar neurons projecting from the nodose ganglia (shown in pink; see panel B). HSV-H129 has a retrograde spread defect. HSV-H129 can spread retrogradelyin first-order neurons but only anterograde spread is observed in second-order pathways. Although HSV immune reactive structures appear similar to those infected by PRV after injection into the ventral stomach (HSV-H129, intact compare with PRV-Bartha), vagal deafferentation (illustrated by a red X on the sensory pathway from the nodose ganglia) eliminates infection of the medial nucleus of the solitary tract and area postrema (HSV-H129, vagal deafferentation). Abbreviations: AMBd, dorsal nucleus ambiguus; AP, area postrema; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; DMV, dorsal motor nucleus of the vagus; IC, insular cortex; LHA, lateral hypothalamic area; mNST, medial nucleus of the solitary tract; mPFC, medial prefrontal cortex; nCen, nucleus centralis of the medial solitary nucleus; NG, nodose ganglion; PVN, paraventricular nucleus of the hypothalamus. (Figure modified from reference 386 with permission of the publisher.)