Abstract
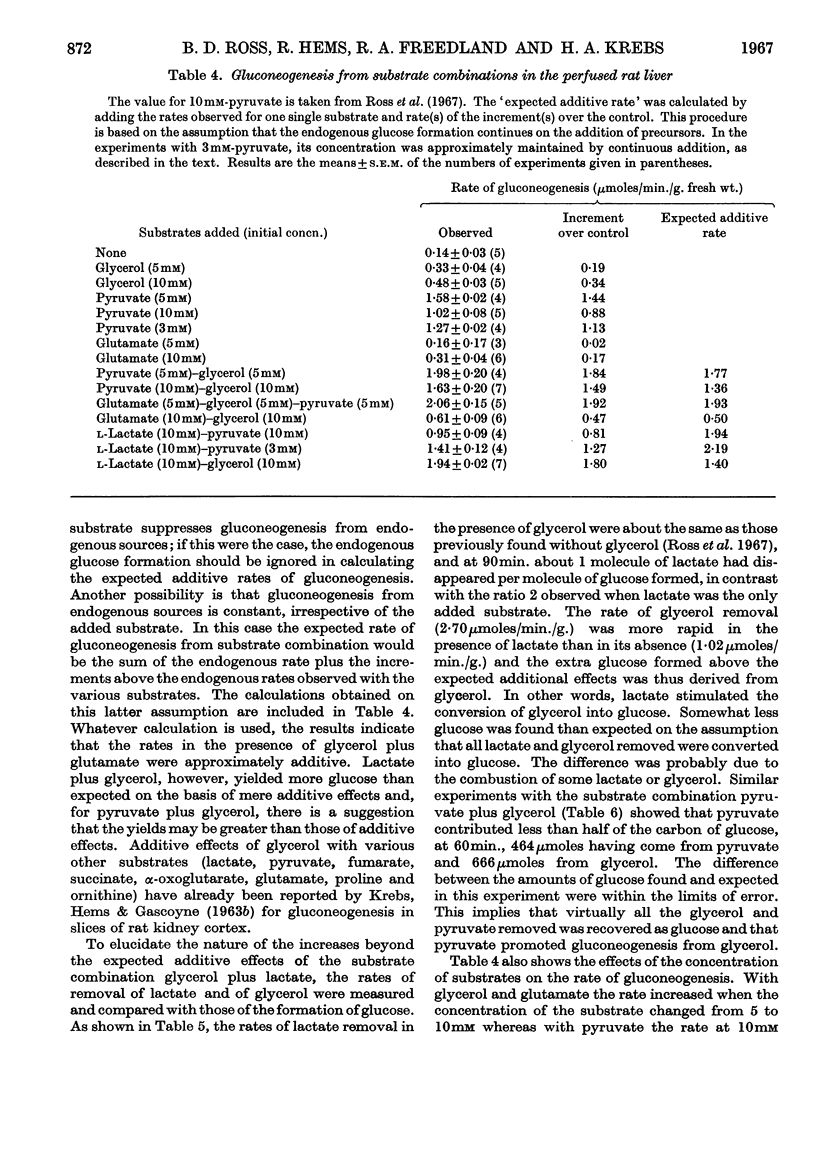
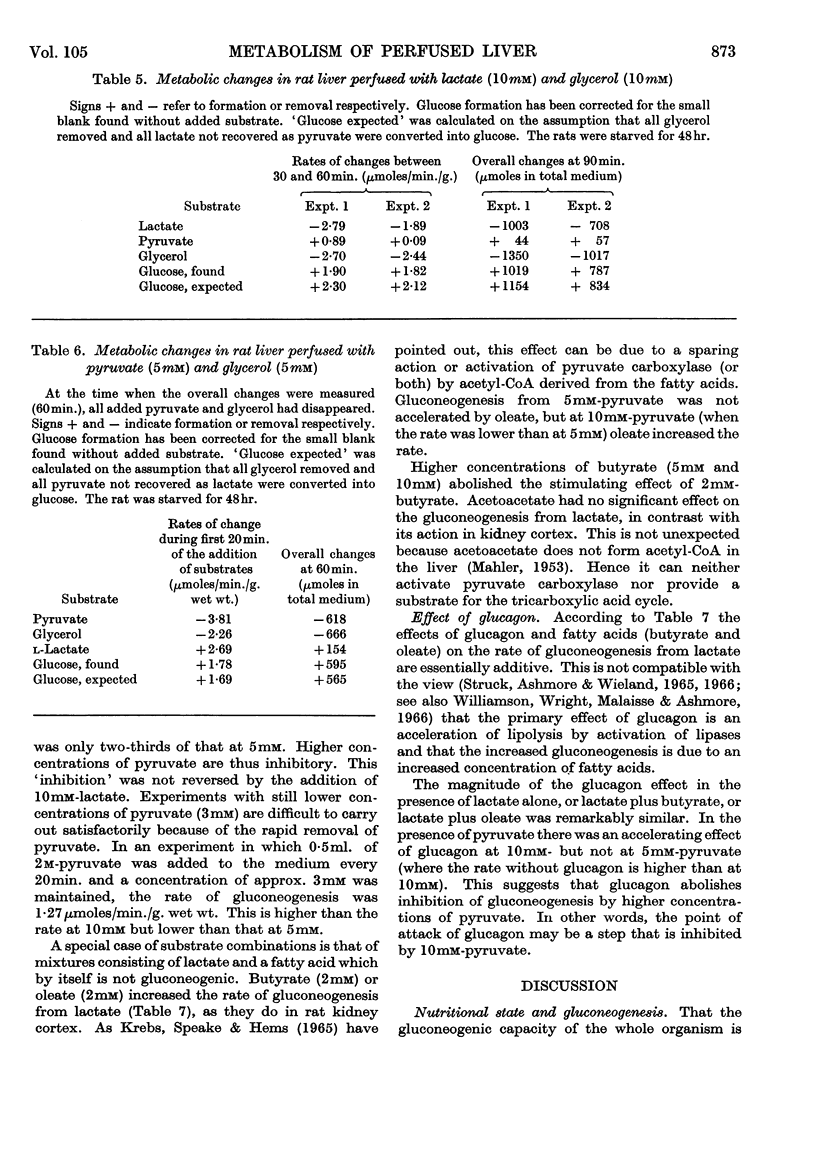
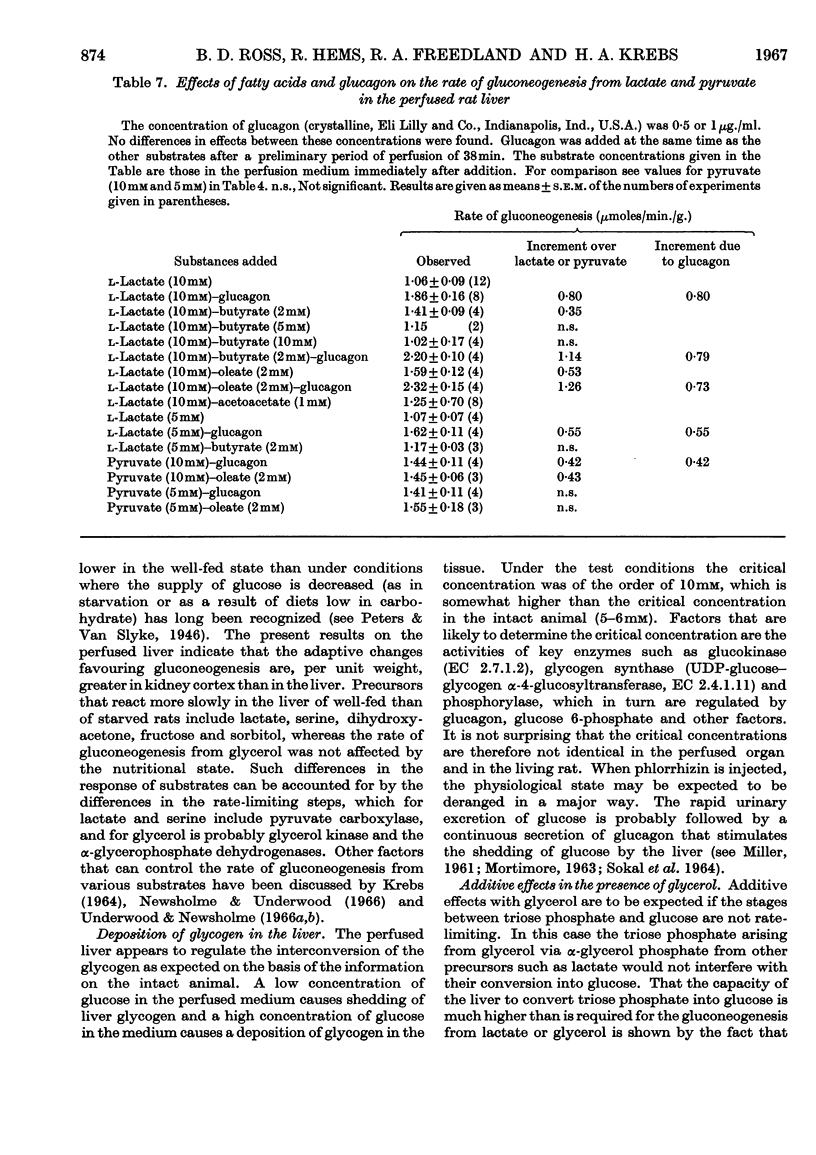
1. The rates of gluconeogenesis from most substrates tested in the perfused livers of well-fed rats were about half of those obtained in the livers of starved rats. There was no difference for glycerol. 2. A diet low in carbohydrate increased the rates of gluconeogenesis from some substrates but not from all. In general the effects of a low-carbohydrate diet on rat liver are less marked than those on rat kidney cortex. 3. Glycogen was deposited in the livers of starved rats when the perfusion medium contained about 10mm-glucose. The shedding of glucose from the glycogen stores by the well-fed liver was greatly diminished by 10mm-glucose and stopped by 13·3mm-glucose. Livers of well-fed rats that were depleted of their glycogen stores by treatment with phlorrhizin and glucagon synthesized glycogen from glucose. 4. When two gluconeogenic substrates were added to the perfusion medium additive effects occurred only when glycerol was one of the substrates. Lactate and glycerol gave more than additive effects owing to an increased rate of glucose formation from glycerol. 5. Pyruvate also accelerated the conversion of glycerol into glucose, and the accelerating effect of lactate can be attributed to a rapid formation of pyruvate from lactate. 6. Butyrate and oleate at 2mm, which alone are not gluconeogenic, increased the rate of gluconeogenesis from lactate. 7. The acceleration of gluconeogenesis from lactate by glucagon was also found when gluconeogenesis from lactate was stimulated by butyrate and oleate. This finding is not compatible with the view that the primary action of glucagon in promoting gluconeogenesis is an acceleration of lipolysis. 8. The rate of gluconeogenesis from pyruvate at 10mm was only 70% of that at 5mm. This `inhibition' was abolished by oleate or glucagon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GORDON E. R. Glucose consumption by the perfused, isolated rat liver. Can J Biochem Physiol. 1963 Jul;41:1611–1620. [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R., GASCOYNE T. RENAL GLUCONEOGENESIS. IV. GLUCONEOGENESIS FROM SUBSTRATE COMBINATIONS. Acta Biol Med Ger. 1963;11:607–615. [PubMed] [Google Scholar]
- KREBS H. A., SPEAKE R. N., HEMS R. ACCELERATION OF RENAL GLUCONEOGENESIS BY KETONE BODIES AND FATTY ACIDS. Biochem J. 1965 Mar;94:712–720. doi: 10.1042/bj0940712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. THE CROONIAN LECTURE, 1963. GLUCONEOGENESIS. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:545–564. doi: 10.1098/rspb.1964.0019. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Notton B. M., Hems R. Gluconeogenesis in mouse-liver slices. Biochem J. 1966 Dec;101(3):607–617. doi: 10.1042/bj1010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHLER H. R. Role of coenzyme A in fatty acid metabolism. Fed Proc. 1953 Sep;12(3):694–702. [PubMed] [Google Scholar]
- MILLER L. L. Some direct actions of insulin, glucagon, and hydrocortisone on the isolated perfused rat liver. Recent Prog Horm Res. 1961;17:539–568. [PubMed] [Google Scholar]
- Ross B. D., Hems R., Krebs H. A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967 Mar;102(3):942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOKAL J. E., MILLER L. L., SARCIONE E. J. Glycogen metabolism in the isolated liver. Am J Physiol. 1958 Nov;195(2):295–300. doi: 10.1152/ajplegacy.1958.195.2.295. [DOI] [PubMed] [Google Scholar]
- SOKAL J. E., SARCIONE E. J., HENDERSON A. M. RELATIVE POTENCY OF GLUCAGON AND EPINEPHRINE AS HEPATIC GLYCOGENOLYTIC AGENTS: STUDIES WITH THE ISOLATED PERFUSED RAT LIVER. Endocrinology. 1964 Jun;74:930–938. doi: 10.1210/endo-74-6-930. [DOI] [PubMed] [Google Scholar]
- Struck E., Ashmore J., Wieland O. Effects of glucagon and long chain fatty acids on glucose production by isolated perfused rat liver. Adv Enzyme Regul. 1966;4:219–224. doi: 10.1016/0065-2571(66)90016-1. [DOI] [PubMed] [Google Scholar]
- Struck E., Ashmore J., Wieland O. Stimulierung der Gluconeogenese durch langkettige Fettsäuren und Glucagon. Biochem Z. 1965 Nov 5;343(1):107–110. [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F., KEECH D. B. PYRUVATE CARBOXYLASE. I. NATURE OF THE REACTION. J Biol Chem. 1963 Aug;238:2603–2608. [PubMed] [Google Scholar]
- Williamson J. R., Wright P. H., Malaisse W. J., Ashmore J. Control of gluconeogenesis by acetyl CoA in rats treated with glucagon and anti-insulin serum. Biochem Biophys Res Commun. 1966 Sep 8;24(5):765–770. doi: 10.1016/0006-291x(66)90391-3. [DOI] [PubMed] [Google Scholar]


