Abstract
Non-invasive respiratory support (NRS), including high flow nasal oxygen therapy, continuous positive airway pressure and non-invasive ventilation, is a cornerstone in the management of critically ill patients who develop acute respiratory failure (ARF). Overall, NRS reduces the work of breathing and relieves dyspnea in many patients with ARF, sometimes avoiding the need for intubation and invasive mechanical ventilation with variable efficacy across diverse clinical scenarios. Nonetheless, prolonged exposure to NRS in the presence of sustained high respiratory drive and effort can result in respiratory muscle fatigue, cardiovascular collapse, and impaired oxygen delivery to vital organs, leading to poor outcomes in patients who ultimately fail NRS and require intubation. Assessment of patients’ baseline characteristics before starting NRS, close physiological monitoring to evaluate patients’ response to respiratory support, adjustment of device settings and interface, and, most importantly, early identification of failure or of paramount importance to avoid the negative consequences of delayed intubation. This review highlights the role of respiratory monitoring across various modalities of NRS in patients with ARF including dyspnea, general respiratory parameters, measures of drive and effort, and lung imaging. It includes technical specificities related to the target population and emphasizes the importance of clinicians’ physiological understanding and tailoring clinical decisions to individual patients’ needs.
Keywords: Non-invasive respiratory support, Acute respiratory failure, Respiratory monitoring
Introduction
Acute respiratory failure (ARF) is one of the leading causes of intensive care unit (ICU) admission worldwide [1]. From a pathophysiological perspective, ARF is characterized by hypoxemia, which may occur alongside with hypoventilation and hypercapnia due to ventilatory pump failure, or hypo-normocapnia (isolated acute hypoxemic respiratory failure -AHRF) in the context of direct or indirect lung insults. The result is a cardio-respiratory incapacity to sustain adequate oxygen delivery to vital organs and to eliminate CO2, contributing to acid–base imbalance [2, 3]. Despite supplemental oxygen being sometimes sufficient to overcome these abnormalities, the increased ventilatory demands may require escalation to more advanced respiratory support [1].
Non-invasive respiratory support (NRS) strategies can elicit a physiological response sufficient to meet patients’ needs during acute illness, potentially avoiding intubation and invasive mechanical ventilation (IMV). However, especially in sicker patients, delaying IMV initiation may expose the patient to excessive respiratory effort and increased oxygen consumption, further aggravating patients’ condition and overall clinical outcomes. As a result, deciding whether to implement NRS, how, and for how long are key clinical decisions.
In this review, we provide an overview of the relevance and available tools for monitoring adult patients during NRS for ARF. We specifically focus on the physiological effects and main indications for NRS, importance of device/interface selection, determinants, and implications of NRS failure. Finally, we describe available tools for monitoring and how to interpret them in the clinical context to guide decisions related to adjustments of ventilator settings and timely intubation to avoid harm. NRS during awake prone positioning and post-extubation are out of the scope of this review.
Physiological effects of non-invasive respiratory support
NRS includes High-Flow Nasal Oxygen (HFNO), Continuous Positive Airway Pressure (CPAP), and Non-Invasive Ventilation (NIV). To adequately interpret monitoring parameters, it is crucial to understand the physiological effects of each strategy.
HFNO provides heated and humidified fresh gas at a high flow rate (30–80 L/min) through a special nasal cannula with a set fraction of inspired oxygen (FiO2) from 0.21 to 1.00 [4]. The gas delivered at high flow rates reduces nasal and upper airway inspiratory resistance while increasing expiratory resistance [5, 6]. As a result, HFNO decreases room air entrainment ensuring stable FiO2 delivery, increases end-expiratory lung volume, generating small amounts of positive end-expiratory pressure (PEEP) (1 to 7 cmH2O varying with settings and conditions) homogenizing ventilation distribution and improving oxygenation [6, 7]. Lastly, delivery of fresh gas results in CO2 wash-out reducing anatomical dead space and decreasing CO2 rebreathing [8]. These effects result in a reduced inspiratory effort and respiratory rate in many patients, particularly those with high ventilatory demands [5, 7].
CPAP maintains a constant PEEP level throughout the breathing cycle. It can act as a mechanical stent for the upper airways and increase end-expiratory lung volume with or without alveolar recruitment [9]. When NIV is applied, inspiratory pressure support (PS) is added above the set PEEP. Both CPAP and NIV can reduce work of breathing, improve respiratory mechanics, and gas exchange [10]. They can be applied through different interfaces, i.e., facemask and helmet. The latter can reduce leaks and discomfort, allowing for higher PEEP as compared to facemasks [11, 12]. Active humidification and heating of inhaled gas may enhance patients’ tolerance and increase the chances of success [13].
Main indications for NRS
Based on these physiological principles and the available clinical evidence, HFNO is currently recommended as first-line therapy in AHRF. Helmet NIV/CPAP remains a reasonable alternative with supporting evidence available for more severe patients with AHRF (PaO2/FiO2 150—200 mmHg) [11, 14, 15]. Extensive data supports the use of CPAP/NIV delivered via facemask or helmet interfaces in patients with cardiogenic pulmonary edema (CPE) due to the beneficial effects of positive pressure in this context (i.e., reduced pre- and afterload) [16–20]. Studies showing the benefit of HFNO were also performed for CPE and it can be used as an alternative when CPAP and NIV are not tolerated/available. [18]. Bilevel facemask NIV remains the first line respiratory support modality for hypercapnic exacerbation of chronic obstructive pulmonary disease (COPD) over helmet NIV and CPAP. [18, 21]. Some studies have shown promising results using HFNO in these patients [17, 21–23], however its use should be reserved only for patients with NIV intolerance in the acute setting. Patients with chest trauma might benefit primarily from facemask or helmet NIV/CPAP [18, 24]. Of note, in a retrospective cohort study HFNO has shown to be better tolerated and leading to equivalent success rates when compared to NIV in these patients [25]. Clinical guidelines recommend the use of CPAP/NIV in immunocompromised patients with ARF over conventional oxygen support [18]. Recent data suggest that helmet might be superior or at least equally effective to facemask CPAP/NIV [26–28]. Furthermore, HFNO can also be considered an alternative in these settings, given that some studies have reported similar results when compared to NIV [29].
Who may not be a good candidate for NRS?
Patients with more severe systemic disease at baseline (e.g., vasopressor need, multiple organ failure) and/or lung injury are less likely to benefit from NRS but rather should be considered for IMV if within patients’ goals of care. Older patients and those with higher SAPS II, non-respiratory sequential organ failure assessment (SOFA) score on admission, greater number of quadrants affected on chest X-ray, diagnosis of pneumonia or severe ARDS (PaO2/FiO2 < 100 mmHg), with concomitant immunosuppression, lower Glasgow Coma Scale, and need of vasoactive drugs have more chances to fail NRS [30–36].
An important caveat of NRS is that, despite unloading the respiratory muscles, there is still some energy expenditure due to respiratory muscles’ contraction which might further compromise end-organ function in critically ill patients [37]. Additionally, in the context of more severe lung injury and systemic inflammation, theoretical risk of P-SILI and myotrauma increases [38]. If for any reason clinicians decide to undergo a trial of NRS in these patients, very close monitoring should be implemented and early consideration to transition to IMV should be consider if there is a lack of initial positive response.
Relevance of interface selection and settings
Interface selection during NRS is important. Patients with AHRF and a PaO2/FiO2 between 150 and 200 mmHg will likely benefit from HFNO as first-line therapy as shown in a randomized clinical trial (RCT) when compared to facemask NIV and conventional oxygen therapy [14]. In this context, asymmetrical cannulas, larger prong/nare diameter relationship, highest tolerable set flow (ideally 50–60 L/min) and individualized temperature selection may boost physiological benefits and comfort of HFNO [4, 7, 39–42]. Helmet NIV could be an alternative to HFNO during AHRF as it elicits a similar physiological response and was shown to be superior to facemask NIV in a different RCT [12, 15]. In addition, helmet CPAP offers the option to be implemented without the need of a mechanical ventilator [43]. In the remaining clinical scenarios, oronasal or full-face masks can be used interchangeably. Full-face interfaces offer an even distribution of pressure over the skin and are not applied over the nasal bridge but increase the chances of claustrophobia as compared to oronasal masks. Setting-up appropriate humidification is crucial. Heat-moisture exchangers have shown similar results in terms clinical outcomes than active humidification during NIV [44], but might impose higher work of breathing and lose efficacy when large leaks are present. Specific recommendations regarding other circuit set-up and NRS settings are described elsewhere [45–48].
Importantly, close monitoring of interface adequacy and comfort is crucial until patients’ stability is reached. In some cases, change in the interface might be required in addition to frequent adjustments of ventilator settings.
What are the determinants and implications of NRS failure?
NRS failure is epidemiologically defined as the subsequent need for endotracheal intubation. However, the decision to intubate is inherently based on clinical judgment, influenced by factors such as healthcare teams’ experience, local practices, and patient-specific considerations, all of which can vary significantly across ICUs. Therefore, clinical significance of “NRS failure” can also be greatly different across settings. In fact, observational data suggest that intubation based on pathophysiological thresholds are scarcely implemented in clinical practice when patients are receiving facemask NIV [49, 50].
Pathophysiological link between NRS failure with delayed intubation and worse clinical outcomes is not clear. At least three possible pathways warrant consideration as potential mediators in the putative causal relationship between NRS failure with delayed intubation and worse clinical outcomes: (1) direct harm to the lung and respiratory muscles from excessive breathing effort in the context of acute illness (i.e., patient self-inflicted lung injury -P-SILI- and myotrauma [51–54]; (2) progression of underlying illness without sufficient unloading of the respiratory muscles and low oxygen delivery to vital organs; (3) complications related to intubation and IMV such as sedation [55], immobilization, diaphragm disuse atrophy, sleep disorders [56], ventilator-induced lung injury [57]. Importantly, the latter mechanism would only be relevant for the potential causal relationship between NRS failure, need for IMV and worse clinical outcomes and not necessarily related to the timing of intubation relative to failure, as complications of IMV are common to both early and delayed intubation. Therefore, monitoring baseline characteristics to ensure a more appropriate selection of candidates for NRS, the magnitude of breathing effort, and the trajectory of illness severity during NRS are important to minimize the risk of harm to the lung, diaphragm, and associated decrease oxygen delivery to vital organs.
How to monitor the response to NRS?
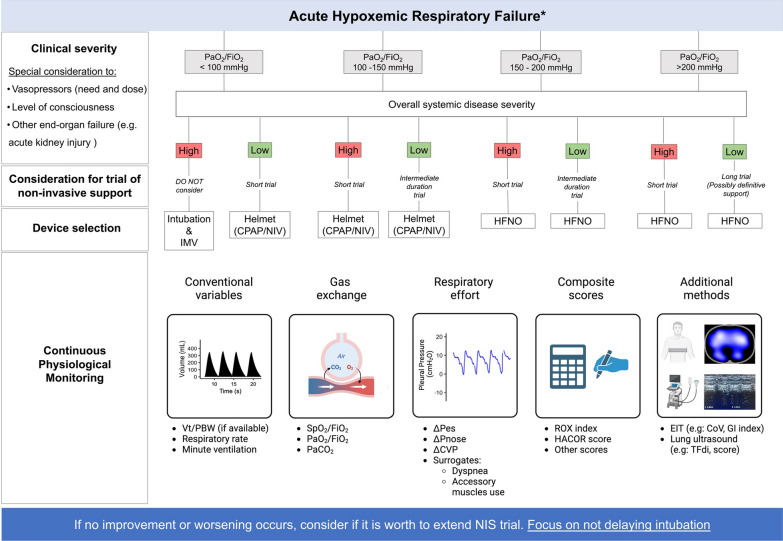
To minimize the risk of failure, a pragmatic approach to implement NRS in patients with AHRF is proposed as a guide focusing on monitoring before and during NRS (Fig. 1). This algorithm is intended to guide decisions in patients with inflammatory conditions leading to AHRF or ARDS. It does not entirely apply for CPE or postoperative patients, where different information might be incorporated into clinical judgment.
Fig. 1.
Integration of baseline characteristics and monitoring tools during non-invasive respiratory support. A simple algorithm is proposed based on baseline characteristics (i.e., oxygenation and clinical severity) to decide on the appropriateness of a trial of non-invasive respiratory support. Available tools to monitor response are also summarized. Duration of short trial and trial of intermediate duration depends on patients’ individual response to therapy, authors suggest considering 1–2 h for a short trial and 3–6 h for a trial of intermediate duration. * intended to guide decisions in patients with acute hypoxemic respiratory failure of infectious etiology (e.g., Community acquired pneumonia) or ARDS. PaO2/FiO2 ratio of arterial partial pressure of oxygen to fraction of inspired oxygen; IMV invasive mechanical ventilation; CPAP continuous positive airway pressure; NIV non-invasive ventilation; HFNO high flow nasal oxygen; Vt/PBW ratio of tidal volume to predicted body weight; SPO2/FiO2 ratio of oxygen saturation to fraction of inspire oxygen; PaCO2 arterial partial pressure of carbon dioxide; ΔPes esophageal pressure swing; ΔPnose swing in nasal pressure; ΔCVP swing in central venous pressure; EIT electrical impedance tomography; CoV center of ventilation; GI index inhomogeneity index; TFdi thickening fraction of the diaphragm
The goals of respiratory monitoring during NRS are to (1) evaluate treatment response, (2) guide adjustments in ventilator settings ensuring adequacy to patients’ ventilatory demands and breathing pattern (e.g., increase/decrease support and correct asynchronies), and, importantly, (3) identify early which patients may benefit from IMV. A multimodal approach should integrate patients’ initial clinical characteristics and trajectory focusing on illness severity, measures of respiratory effort, gas exchange, and other variables including lung imaging (Fig. 1—Table 1).
Table 1.
Monitoring variables
| Category | Variable | Complexity | NRS device evaluated | Thresholds for NRS failure | Comments | Caveats |
|---|---|---|---|---|---|---|
| Conventional variables | Tidal Volume (Vt) | + | Facemask NIV | > 9–9.5 mL/kg PBW after 1 h | Indirect estimate of respiratory effort and stress | Influenced by leaks, mechanics and settings. Not feasible with helmet, HFNO or single-limb circuits |
| Respiratory Rate (RR) | + | Facemask NIV—HFNO | > 25 bpm or lack of improvement | Widely used | Poor measure of drive. Influenced by anxiety, pain, discomfort. Sensitive to lack of HFNO response | |
| Minute Ventilation | + | Facemask NIV | > 11 L/min | Composite of Vt and RR | Idem Vt and RR | |
| Respiratory effort | Esophageal Pressure Swing (ΔPes) | + + + | NIV—HFNO | > 10 cmH2O after 2 h | Gold standard for inspiratory effort | Expertise and time for catheter insertion. Complex validation during NRS |
| Central Venous Pressure Swing (ΔCVP) | + + + | Helmet NIV—facemask CPAP | Unknown | Alternative to ΔPes for high effort | Requires CVP catheter and lack of arrhythmia. Lacks clinical validation | |
| Nasal Pressure Swing (ΔPnose) | + + | HFNO | > 5 cmH2O after 2 h | Minimally invasive and correlated with ΔPes | Custom-made equipment connected to pressure transducer | |
| Dyspnea | + | NIV | ≥ 4 points in VAS | Associated with effort and clinical outcomes | Communicative patients | |
| Gas exchange | PaO2/FiO2 | + + | Facemask NIV | < 200 mmHg after 1 h and/or worsening | Overall marker of severity and response to treatment | Influenced by extrapulmonary factors (hemodynamics, drugs) |
| SpO2/FiO2 | + | HFNO | < 113–115 in the first 12 h or worsening | Overall marker of severity and response to treatment | SpO2 valid if SpO2 < 97%. Affected by skin color, perfusion and temperature | |
| Carbon Dioxide | + + | Facemask NIV | < 32 mmHg prior to NIV | Hypocapnia may reflect high effort | Hypocapnia may be absent with high dead space / shunt | |
| Composite scores | HACOR (heart rate, acidosis, consciousness, oxygenation, respiratory rate) | + + | Facemask NIV—HFNO | > 5 points after 1 h | – | Requires arterial blood gases |
| ROX (SpO2/FiO2 to RR ratio) | + | Facemask CPAP—HFNO | < 4.88 after 6–12 h (HFNO); < 6.64 after 24 hs (CPAP) | – | Idem SpO2/FiO2 and RR | |
| BREF (Base excess RR, PaO2/FiO2.) | + + | HFNO, helmet, and facemask CPAP/NIV | Unknown | To estimate ΔPes > 10 cmH2O in non-intubated patients receiving NRS | Arterial blood gases | |
| Other methods | Ultrasound | + + | Facemask and Helmet NIV—HFNO | LUS score ≥ 12; LUS areas ≥ 5 points; Thickening fraction < 36.3% | Lung aeration and diaphragmatic function | Operator dependent |
| Electrical Impedance Tomography | + + + | HFNO | Unknown | Regional ventilation and perfusion | Not widely available, expensive. Expertise and additional offline analysis |
A summary of most important monitoring tools and variables that can be used during NRS is detailed in Tables 1 and 2, highlighting technical characteristics, clinical interpretation of findings, caveats, and suggested adjustments based on results.
Table 2.
Physiological consideration for suggested interventions based on monitoring
| Variable | Potential physiological mechanism | Consider interventions |
|---|---|---|
| RR increase | High drive | ↑ PS, ↑ PEEP, ↑ flow rate (HFNO), ↑ FiO2 to SpO2 target 98% |
| When combined with low Vte, loss of lung aeration | ↑ PEEP, ↑ PS, ↑ flow rate (HFNO), optimize body position | |
| Neuromuscular uncoupling by excessive PEEP | ↓ PEEP | |
| Anxiety, discomfort, agitation | Trial of different interface, conscious sedation*, and analgesia | |
| RR decrease | Adequate drive, positive response to NRS | Evaluate progressive withdrawal of PS / PEEP, flow rate |
| With ineffective efforts, overassistance | ↓ PS, set FiO2 to a lower SpO2 target (90–95%) | |
| Excessive opioids or sedatives | Reduction in drug dose | |
| Together with other signs of fatigue, imminent respiratory arrest | Intubate immediately | |
| High Vte | High effort | ↑ PEEP, ↑ flow rate to maximum tolerable (ideally 60 L/min—HFNO), ↑ FiO2 to SpO2 target 98%, consider helmet NIV, consider IMV |
| When combined with low RR, opioid intoxication | Consider opioid dose reduction | |
| Excessive PS | ↓ PS | |
| Low Vte | Adequate effort, positive response to NIV | Consider weaning |
| Low effort, excessive sedative dose | Reduction of sedatives | |
| Low compliance due to lung aeration loos | ↑ PEEP, optimize body position | |
| High or increase in PaO2/FiO2 | Lung recruitment | Progressive withdrawal of respiratory support—Consider weaning |
| Low proportion of low V/Q—shunt units (↓ severity) | Consider weaning | |
| Low or decrease in PaO2/FiO2 | High proportion of low V/Q—shunt units (↑ severity) | ↑ PEEP, optimize body position, consider helmet interface |
| High PaCO2 | High proportion of high V/Q—dead space units (↑ severity) | ↑ PS, revaluate PEEP level |
| Decreased muscular performance (weakness, excessive PEEP) | ↑ PS, reconsider PEEP level | |
| Low PaCO2 | High effort | Escalate NRS to helmet, ↑ PS, reconsider PEEP level, consider IMV |
| Low proportion of high V/Q—dead space units (↓ severity) | Consider weaning |
*conscious sedation: a trial of dexmedetomidine infusion (for agitation), low dose opioid (for dyspnea/pain) administration can be considered cautiously and with close monitoring
Dyspnea and comfort
Dyspnea and discomfort should be regularly assessed during NRS. First, dyspnea frequently occurs in the context of and is associated with high respiratory drive [58, 59] Additionally, dyspnea intensity has been independently associated with a greater risk of intubation and mortality among spontaneously breathing patients with ARF and therefore these patients merit closer monitoring [60, 61]. Finally, intolerance and discomfort related to the NIV interface are closely associated with NIV failure highlighting the importance of appropriate interface selection and fitting [34, 35]. In general, better comfort was reported with HFNO and helmet NIV as compared to facemask NIV. Importantly, while using facemask interface, the need for adjustments is frequent as well as resting periods, requiring alternating support with a different interface/device (e.g., HFNO).
The preferential method to measure dyspnea in conscious communicative patients is the self-reported visual analog scale (VAS), quantifying dyspnea as a continuous variable. The patient points to a vertical line representing their dyspnea intensity on a horizontal line ranging from 0 to 100 mm (lack of dyspnea to maximum dyspnea). Alternatively, a simpler numeric-rating scale (NRS) can be used ranging from 0 to 10 using numbers or representative figures [62]. VAS and NRS can be implemented to assess also patients’ comfort during NRS.
Basic variables
Expired tidal volume (Vte) can be monitored during NIV/CPAP. Because it represents the output of respiratory effort and a determinant of lung stress, it is often considered an indirect estimate of those variables. In fact, a high Vte (≥ 9.5 mL/Kg of predicted body weight [PBW]) has been consistently associated with failure and overall worse outcomes during facemask NIV in patients with moderate-to-severe AHRF [63–65]. However, clinical interpretation of a high Vte during NIV/CPAP should consider the physiological context. First, variables different than the respiratory effort can also influence the monitored Vte, e.g., high PS associated with high respiratory system compliance can lead to high Vte [66, 67]. However, an increase in Vte without changes in ventilator settings and assuming stability of respiratory system mechanics within a relatively short time is usually indicative of an increase in respiratory effort either because of a high respiratory drive or improvement in neuromuscular coupling. Second, setting PS to target high Vte (10 to 15 mL/Kg of PBW) has recently shown to reduce the number of patients with a hypercapnic exacerbation of COPD requiring intubation [68]. This finding suggests that high PS with high Vt may be effective in relieving dyspnea and effort without added risk of VILI in a subgroup of patients; however, in all cases close monitoring is advised. Specifically, occurrence of ineffective efforts is frequent in the context of overassistance (i.e., excessive support) in patients with auto-PEEP, therefore careful inspection of ventilator waveforms is recommended. Finally, leaks, associated with other asynchronies and often intolerance, are also frequent with higher support and should be avoided [69].
Some technical considerations are important when monitoring Vte during NRS. With helmet CPAP/NIV, quantifying Vte accurately is not feasible because part of the insufflated volume is used to distend the interface. Similarly, dedicated NIV ventilators with single-limb configurations and intentional leak do not measure but calculate Vte based on predefined algorithms, thus the accuracy of Vte estimation might be worse than with double-limb ICU ventilators [70–72]. In any case, when monitoring Vte, leaks should be minimized. During HFNO, Vt monitoring is not widely available for clinical use, dynamic changes in lung impedance measured with electrical impedance tomography (EIT) could theoretically be used for longitudinal follow-up, but validation is missing [73–75].
Respiratory rate (RR) remains one of the most monitored variables during NRS. Clinicians often associate a high RR with high respiratory drive and effort; however, it is often a late sign of high respiratory drive [76]. High RR is common in critically ill patients and can reflect other factors such as systemic inflammation, anxiety, pain, discomfort and abnormal respiratory mechanics [77]. Furthermore, ineffective efforts may mask the patients’ true RR when auto-PEEP is present and respiratory muscles’ output is insufficient to trigger the ventilator [69]. Despite these caveats, RR remains a key variable to consider, especially during HFNO as its expected physiological effect on decreasing RR is very strong [5]. A lack of decrease in baseline RR often represents a lack of clinical response, particularly during HFNO. In fact, a high RR and a lack of initial decrease during NRS were shown to predict failure during facemask and helmet CPAP/NIV, and HFNO [33, 78–81]. However, given the complex physiology underlying RR regulation, it is not recommended to make decisions based solely on RR.
As expected, higher minute ventilation, i.e., the product of Vt and RR, was also found to be associated with NIV failure in patients with AHRF and mild ARDS [63, 82].
Gas exchange
Changes in oxygenation are important determinants of NRS success/failure in patients with ARF, partly because they are a marker of severity and clinical response to therapy and are frequently considered as a criterion influencing the decision to intubate. Teasing out the relative importance of each factor is, therefore, challenging.
All NRS strategies, especially those that promote higher airway pressure, can increase oxygenation by multiple mechanisms. In fact, patients with ARF usually experience a transient improvement in oxygenation during NIV/CPAP that can return to baseline after device removal [83]. Importantly, transient improvement in oxygenation during NRS without a clear trend towards clinical improvement might give false reassurance and contribute to poor outcomes in patients’ ultimately failing NRS and receiving delayed intubation [30, 83].
Oxygenation can be monitored both continuously by pulse oximetry and intermittently by arterial blood gases to obtain precise PaO2/FiO2 ratio prior to NRS initiation and after the first 2–6 h of treatment. A lower baseline PaO2/FiO2 and a lack of improvement over time are independent predictors of NIV/CPAP failure [30, 31, 64, 81, 84]. In addition, lower SpO2/FiO2 ratio is associated with HFNO failure at various time points within the first 24 h [33, 85]. However, technical pitfalls related to SpO2 measures should be considered, such as skin color and temperature, hemodynamics and perfusion, anemia and hyperoxia among others [86].
Interpreting levels of partial pressure of carbon dioxide (PaCO2) in the context of AHRF is complex. A relatively low PaCO2 can be indicative of less severe lung injury (lower dead-space/shunt fraction) and lack of respiratory muscle fatigue. However, very low PaCO2 associated with high respiratory drive and effort can be secondary to an exaggerated ventilatory response caused by strong stimuli that overwhelm the patients’ control of breathing [76, 87–90]. In a recent single center study, a PaCO2 lower than 32 mmHg was strongly associated with NIV failure [91]. Additionally, a secondary analysis of a randomized trial showed that patients with more pronounced hypocapnia (< 35 mmHg) benefitted from helmet NIV when compared to HFNO. This effect may be attributed to the higher PEEP and PS provided by helmet, likely reducing respiratory drive and effort more effectively [91, 92].
The absence of hypocapnia does not exclude high drive and effort as high minute ventilation may not be sufficient for CO2 clearance in patients with high dead space/shunt fraction or when respiratory muscles’ exhaustion occurs [76]. An increase in PaCO2 within the first days of NIV was independently associated with NIV failure in ARDS, probably indicating deterioration of lung function (i.e., VILI, P-SILI) and/or respiratory muscles’ performance (i.e., diaphragmatic fatigue, muscle injury) [31].
Monitoring PaCO2 to ensure a downtrend is key during acute hypercapnic respiratory failure. Even though blood samples are often required, transcutaneous monitoring, despite not being widely available, offers an attractive alternative that closely correlates with PaCO2 with a small bias during acute respiratory failure [93].
Respiratory effort
Direct monitoring of breathing effort with esophageal pressure (Pes) offers invaluable information. However, it is challenging to implement in the acute setting. It provides information related to baseline ventilatory demands and it is a direct measure of the physiological response to NRS in terms of respiratory muscles’ unloading and risk of P-SILI. The gold standards for inspiratory effort quantification based on Pes are the muscular pressure (instantaneous effort) and pressure–time product of the respiratory muscles (effort during whole inspiration). However, both parameters require calculations that depend on the estimation of chest wall compliance. Measurement of the tidal swing in Pes (ΔPes) is a good estimate of inspiratory effort and can be easily implemented at the bedside [88]. Risk of P-SILI can be estimated by calculating driving transpulmonary pressure (i.e., the difference between airway and esophageal pressure), allowing to quantify the dynamic lung distending pressures. Specifications about how to perform Pes measurements and calculations are detailed elsewhere [94, 95].
In a prospective observational study, a reduction in ΔPes > 10 cmH2O after 2 h of facemask NIV was found to be the best predictor of NIV success in moderate-to-severe AHRF [65]. Furthermore, ΔPes reduction was positively associated with improvement in radiographic changes within 24 h and 30-day mortality. Despite these findings being exploratory, they support the hypothesis of P-SILI being mediated by the magnitude of breathing effort during NIV and demonstrate the association between worsening lung function following higher breathing effort and poor clinical outcomes. Similarly, in patients during helmet NIV a reduction in ΔPes to values < 10 cmH2O and dynamic lung stress to < 20 cmH2O were associated with a lower need for subsequent intubation [12].
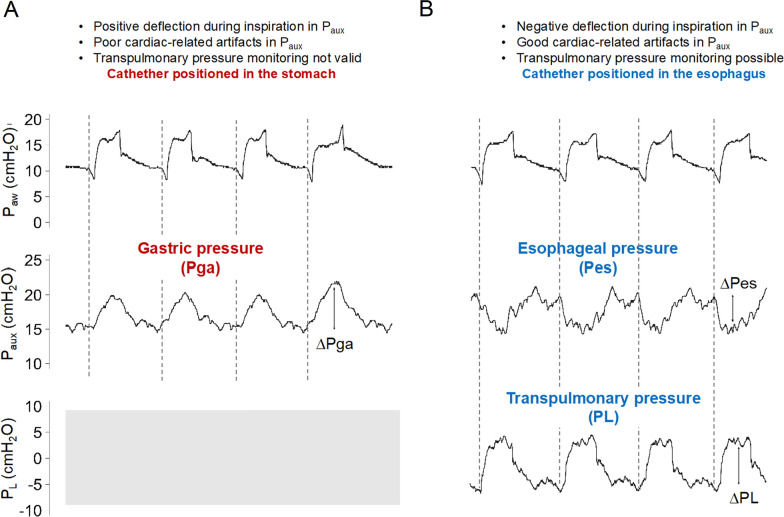
There are technical challenges related to Pes monitoring during NRS. Inserting an esophageal catheter in non-intubated patients with respiratory distress and ensuring adequate positioning by performing an occlusion test to measure ΔPes to delta airway pressure (ΔPaw) ratio can be complex. We offer three alternatives. (1) Aim for a set depth of catheter insertion around 40 cm from the nostril. First, insert esophageal catheter up to around 60 cm and ensure proper inflation of the balloon with the manufacturers’ recommended filling volume, waveform morphology should resemble that of gastric pressure, i.e., positive deflection with tidal, relaxed, inspiration. Then, withdraw the catheter 20 cm and check that waveforms’ morphology resembles that of esophageal pressure, i.e., negative deflection during inspiration and cardiac oscillations (Fig. 2). (2) Use a mouthpiece and a pneumotachograph connected to a stand-alone device to measure simultaneous Paw, flow and Pes and perform a regular occlusion test [96]. (3) Use a tightly fitted facemask with a dual limb circuit connected to the ventilator and perform a regular occlusion test. When measuring an occlusion test, air leaks should be avoided. It is important to emphasize that a proper occlusion maneuver is essential for ensuring a valid estimation of pleural pressure via Pes monitoring.
Fig. 2.
Esophageal catheter insertion during non-invasive respiratory support. Respiratory recordings during non-invasive ventilation through facemask. Airway pressure, auxiliary pressure, and transpulmonary pressure (difference between airway pressure and esophageal pressure) are displayed (top, medium, and bottom respectively). Vertical dotted lines indicate separation between the respiratory cycles. Of note, during high flow nasal oxygen, there is no airway pressure monitoring and identification of individual respiratory cycles, inspiration and expiration require observation of the patient. Panel A) shows the initial position of the catheter in the stomach (approximately 60 cm from the nostril). Gastric pressure (Pga) is recognized by the characteristic positive deflection in auxiliary pressure (Paux) during relaxed inspiration due to the caudal displacement of the diaphragm. Panel B) shows positioning of the catheter in the lower third of the esophagus (approximately 40 cm from the nostril). Paux becomes negative during inspiration and cardiac oscillations become more noticeable. Measurement of esophageal pressure swing (ΔPes) and lung stress (ΔPL) are shown with vertical solid lines. Paw airway pressure; Paux auxiliary pressure; Pga gastric pressure; Pes esophageal pressure; PL transpulmonary pressure
Diaphragmatic ultrasound is a non-invasive technique to monitor inspiratory effort. Quantifying diaphragm thickening fraction (Tfdi) can be done at the zone of apposition with a linear probe positioned on the 8th–9th intercostal space, anterior axillary line [88]. A small prospective study showed that a low Tfdi (< 36%) assessed within the first 96 h of NIV identified patients who failed NIV with acceptable diagnostic accuracy, suggesting that early diaphragmatic dysfunction may play a role in NIV failure [97].
The central venous pressure swing (ΔCVP) has shown good concordance with ΔPes, being useful in identifying strong respiratory effort and titrating PS [98]. A ΔCVP > 15 cmH2O was shown to precisely identify high inspiratory effort in hypoxemic patients during helmet NIV [99]. The main limitation is that central venous catheter insertion is uncommon in patients receiving NRS.
Another minimally invasive technique to monitor inspiratory effort is the nasal pressure swing (ΔPnose) [100]. To monitor ΔPnose, a custom-made catheter covered by a self-expanding foam plug is placed in the same nostril as the nasogastric tube and connected to a pressure transducer (auxiliary port on the ventilator or stand-alone device). The contralateral nostril should be kept patent. When HFNO is used, the cannula is placed only in the patent nostril during monitoring. The ΔPnose has demonstrated excellent correlation with ΔPes during HFNO and facemask NIV [100]. In a cohort study of 102 patients with AHRF receiving HFNO, a ΔPnose > 5.1 cmH2O accurately identified failure and need for escalation in ventilatory support (i.e., use of NIV or IMV) (Area Under the Receiver Operating Curve [AUROC] = 0.98; 95% confidence interval: 0.96–1, P < 0.001) [101].
Another important variable often considered by clinicians is the evidence of non-diaphragmatic inspiratory muscle activation, as it is often indicative of high respiratory drive and effort [102]. Sternocleidomastoid activates when the inspiratory effort is close to 35–40% of the maximum inspiratory pressure, which coincides with thresholds proposed to prevent diaphragmatic fatigue [103, 104]. Activation of other non-diaphragmatic inspiratory muscles, such as intercostals, scalene, and alae nasi increases linearly with inspiratory effort [103, 105]. However, the high interobserver variability and lack of bedside quantitative measure of non-diaphragmatic inspiratory muscle activity remain a challenge. Surface electromyography may help to overcome these limitations in the future [105].
Composite scores
The HACOR score (heart rate, acidosis, consciousness, oxygenation, and RR) was developed and validated in hypoxemic patients receiving facemask NIV. A value higher than 5 after 1 h of NIV was shown to accurately predict failure of facemask NIV (AUROC = 0.89) and helmet CPAP (AUROC = 0.74) [79, 106]. The HACOR score was recently updated and validated incorporating additional baseline clinical variables directly related with a higher risk of failure (e.g., immunosuppression and septic shock), further highlighting the relevance of considering initial disease severity to estimate the risk of failure [107].
The ROX index (SpO2/FiO2 ratio divided by RR) is a simple and widely used clinical score. Threshold values associated with NRS failure vary according to the respiratory support modality and patient population. For HFNO in AHRF, a value lower than 4.88 was associated with increased risk of failure when collected at various timepoints during the first 12–24 h [33]. A ROX index lower than 6 was shown to predict NRS failure in COVID-19 AHRF receiving CPAP, but with acceptable precision only 24 h after CPAP initiation [108]. Finally, in patients receiving facemask NIV, a higher risk of failure was consistently observed with decreasing ROX values (i.e., 23%, 34.1%, 64.3%, and 100% with a ROX index higher than 10, 6–10, 2–6, and lower than 2 after 1–2 h of NIV, respectively) [109]. Moreover, an increase in ROX over time is seen in patients who succeed NRS and stability or lack of improvement in those who ultimately require intubation [33].
Other indices such as the VOX index (Volume Oxygenation—calculated as SpO2/FiO2 to Vt) or composite scales to estimate the inspiratory effort like the BREF score (base excess -B-, respiratory rate -RE-, and PaO2/FiO2 -F-) might be useful when less monitoring tools are available. However, these still require further refinement and validation in prospective larger multicenter cohort studies [85, 110].
Additional tools
Lung ultrasound (LUS) can be used to non-invasively quantify lung aeration as a baseline predictor of success/failure and to monitor response to NRS. In a prospective cohort study in patients with AHRF due to COVID-19, an aeration-based LUS score measured on admission while receiving supplemental oxygen showed acceptable performance to predict helmet NIV and HFNO failure [111]. Additionally, an independent prospective cohort study in the same population has shown that a combination of LUS score and ROX index measured during NIV can accurately predict negative outcomes in these patients [112].
Electrical impedance tomography (EIT), although not widely available, can be used to assess lung aeration and response to NRS, provide a non-invasive, indirect, measure of Vt, and monitor regional ventilation distribution [113]. Only few small single center studies have evaluated the performance of EIT-derived parameters to predict the response to HFNO or NIV in patients with ARF [74, 114, 115]. Overall, these studies show that a more asymmetrical ventilation is potentially associated with higher risk of failure, however, more data are required for bedside translation of the findings [74, 114, 115].
When to define failure based on bedside monitoring
Patients with ARF who receive NRS strategies and are never intubated have better outcomes than those who require IMV, highlighting the relevance of pursuing a trial of NRS in the appropriate patients. However, there is a risk of delaying intubation associated with higher mortality if NRS is prolonged despite a lack of clinical improvement [30]. Although evidence is still conflicting, large observational data suggest that more hypoxemic patients (PaO2/FiO2 < 150 mmHg) initially managed with NIV have worse clinical outcomes than matched patients who receive IMV initially [31]. Currently, there is no consensus regarding maximal duration of NRS trials or clear-cut thresholds for monitored variables used to decide how and when to escalate respiratory support.
Early, i.e., within 1–2 h, improvement or, alternatively, clear worsening might be informative. Interestingly, upward or downward trends in most of the monitoring variables described can already predict failure or success within the first few hours (Table 1). Additionally, high secretion burden, decreased level of consciousness (i.e., Glasgow Coma Scale < 10) and inability to achieve proper interface fit despite sufficient adjustments and trial of different interfaces at any time during NRS therapy should prompt consideration for escalation in respiratory support.
Clinical decision making becomes even more challenging when there is a relative early improvement after starting NRS without additional change within the following 24 h. In this context, fixed time intervals for regular monitoring (e.g., every 4–6 h) are advisable. It is important to distinguish between “delayed intubation” and “late intubation”. While the former refers to a delay between the time of fulfilling intubation criteria (i.e., NRS failure) and intubation, the latter illustrates that intubation occurred at an advanced stage from the initiation of therapy but was not necessarily delayed. Despite the distinction, observational studies have consistently shown that patients with de novo AHRF who failed HFNO or NIV have worse outcomes when intubation is performed beyond 24–48 h of the initial support [30, 79, 116, 117].
Future directions
Identification and prospective validation of thresholds for monitored variables to decide when to escalate respiratory support are needed. Novel study designs such as emulation target trials and collaborative adaptive platforms aim to close this gap [118–120]. In addition, further non-invasive and widely available monitoring tools to assess the respiratory effort (e.g., Pnose) and lung stress may allow to achieve personalized NRS titration. The possibility of monitoring respiratory drive and effort using parameters derived from airway occlusion pressure, i.e., P0.1 [88, 121] and ΔPocc [122] could help to achieve this goal. In this context, an integrated approach considering both drive and effort is essential, as certain clinical circumstances may modify the relationship between respiratory drive and inspiratory effort (e.g., respiratory muscle weakness) [76]. Technical and clinical validation are required, and several research groups are currently performing these studies [123, 124]. One of the main challenges that arise when using these techniques is to control leaks [123]. Under no-leak conditions, high P0.1 during facemask NIV (> 3 cmH2O) was shown to detect respiratory distress shortly after extubation [125]. Besides, standardizing ventilator settings (e.g., CPAP) to evaluate central drive and effort could enable a more accurate and unbiased comparison among patients [124]. Simple bedside techniques to quantify Vt, particularly during helmet NIV/CPAP, and HFNO, are also needed. Even though EIT offers the potential to become a bedside tool for Vt quantification, it currently requires calibration with a known tidal volume measured by other devices (e.g., ventilator) and clinical validation before bedside implementation [43, 113, 126].
Conclusions
Non-invasive respiratory support has consistently shown variable efficacy across different scenarios in preventing the harmful effects of invasive ventilation and improving outcomes for patients with acute respiratory failure. A thorough assessment of the patients’ initial characteristics, combined with close physiological monitoring to adjust settings is essential to tailor a personalized approach and minimize the risk of harm and failure. Importantly, applying non-invasive respiratory support in critically ill patients, particularly those with de novo acute respiratory failure, demands a delicate balance between avoiding invasive ventilation and ensuring timely intubation when clinically indicated.
Acknowledgements
Not applicable.
Abbreviations
- AHRF
Acute hypoxemic respiratory failure
- ARDS
Acute respiratory distress syndrome
- ARF
Acute respiratory failure
- AUROC
Area under the receiver operating curve
- Bpm
Breaths per minute
- BREF
Base excess, respiratory rate, PaO2/FiO2
- COPD
Chronic obstructive pulmonary disease
- CoV
Center of ventilation
- CPAP
Continuous positive airway pressure
- CPE
Cardiogenic pulmonary edema
- ΔCVP
Delta central venous pressure
- ΔPes
Delta esophageal pressure
- ΔPL
Driving transpulmonary pressure
- ΔPnose
Delta nasal pressure
- ΔPocc
Delta oclussion pressure
- EIT
Electrical impedance tomography
- FiO2
Fraction of inspired oxygen
- GI index
Global inhomogeneity index
- HACOR
Heart rate, acidosis, consciousness, oxygenation, respiratory rate
- HFNO
High flow nasal oxygen
- ICU
Intensive care unit
- IMV
Invasive mechanical ventilation
- LUS
Lung ultrasound
- NIV
Non-invasive bilevel positive-pressure ventilation
- NRS
Non-invasive respiratory support
- P0.1
Airway pressure decay at 100 ms
- PaCO2
Arterial pressure of carbon dioxide
- PaO2
Arterial pressure of oxygen
- Paux
Auxiliary pressure
- PEEP
Positive end-expiratory pressure
- Pes
Esophageal pressure
- Pga
Gastric pressure
- PL
Transpulmonary pressure
- PS
Pressure support
- P-SILI
Patient self-inflicted lung injury
- ROX
Ratio of SpO2/FiO2 to respiratory rate
- RR
Respiratory rate
- SpO2
Pulse oxygen saturation
- TFdi
Thickening fraction of diaphragm
- VAS
Visual-analog scale
- VILI
Ventilator-induced lung injury
- Vt/PBW
Tidal volume to
Author contributions
JP, LB, and IT contributed to the study concept and design, literature research process, acquisition, critical appraisal and interpretation of data. All the authors contributed to drafting the manuscript and critically revising it for important intellectual content. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
IT reports personal fees from Medtronic and MbMed SA not related to the current work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA J Am Med Assoc. 2016;315:788–800. [DOI] [PubMed] [Google Scholar]
- 2.Levy MM. Pathophysiology of oxygen delivery in respiratory failure. Chest. 2005;128:547S-553S. [DOI] [PubMed] [Google Scholar]
- 3.Lorente JA, Renes E, Gómez-Aguinaga MA, Landín L, de la Morena JL, Liste D. Oxygen delivery-dependent oxygen consumption in acute respiratory failure. Crit Care Med. 1991;19:770–5. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Albuainain FA, Tan W, Scott JB, Roca O, Mauri T. The effects of flow settings during high-flow nasal cannula support for adult subjects: a systematic review. Crit Care. 2023;27:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vieira F, Bezerra FS, Coudroy R, Schreiber A, Telias I, Dubo S, et al. High-flow nasal cannula compared with continuous positive airway pressure: a bench and physiological study. J Appl Physiol. 2022;132:1580–90. [DOI] [PubMed] [Google Scholar]
- 6.Goligher EC, Slutsky AS. Not just oxygen? Mechanisms of benefit from high-flow nasal cannula in hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195:1128–31. [DOI] [PubMed] [Google Scholar]
- 7.Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195:1207–15. [DOI] [PubMed] [Google Scholar]
- 8.Möller W, Feng S, Domanski U, Franke K-J, Celik G, Bartenstein P, et al. Nasal high flow reduces dead space. J Appl Physiol. 2017;122:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macintyre NR. Physiologic effects of noninvasive ventilation. Respir Care. 2019;64:617–28. [DOI] [PubMed] [Google Scholar]
- 10.Munshi L, Mancebo J, Brochard LJ. Noninvasive respiratory support for adults with acute respiratory failure. N Engl J Med. 2022;387:1688–98. [DOI] [PubMed] [Google Scholar]
- 11.Menga LS, Delle Cese L, Rosà T, Cesarano M, Scarascia R, Michi T, et al. Respective effects of helmet pressure support, continuous positive airway pressure, and nasal high-flow in hypoxemic respiratory failure: a randomized crossover clinical trial. Am J Respir Crit Care Med. 2023;207:1310–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grieco DL, Menga LS, Raggi V, Bongiovanni F, Anzellotti GM, Tanzarella ES, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2020;201:303–12. [DOI] [PubMed] [Google Scholar]
- 13.Hernández G, Paredes I, Moran F, Buj M, Colinas L, Rodríguez ML, et al. Effect of postextubation noninvasive ventilation with active humidification vs high-flow nasal cannula on reintubation in patients at very high risk for extubation failure: a randomized trial. Intens Care Med. 2022;48:1751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–96. [DOI] [PubMed] [Google Scholar]
- 15.Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome a randomized clinical trial. JAMA J Am Med Assoc. 2016;315:2435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan L, Lu Y, Deng M, Zhang Q, Bian Y, Zhou X, et al. Efficacy of high-flow nasal cannula in patients with acute heart failure: a systematic review and meta-analysis. BMC Pulm Med. 2023;23:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RENOVATE Investigators and the BRICNet Authors, Maia IS, Kawano-Dourado L, Tramujas L, de Oliveira NE, Souza RN, et al. High-Flow nasal oxygen vs noninvasive ventilation in patients with acute respiratory failure: the RENOVATE randomized clinical trial. JAMA [Internet]. 2024; Available from: http://www.ncbi.nlm.nih.gov/pubmed/39657981 [DOI] [PMC free article] [PubMed]
- 18.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50:1602426. [DOI] [PubMed] [Google Scholar]
- 19.Tonnelier J-M, Prat G, Nowak E, Goetghebeur D, Renault A, Boles JM, et al. Noninvasive continuous positive airway pressure ventilation using a new helmet interface: a case-control prospective pilot study. Intens Care Med. 2003;29:2077–80. [DOI] [PubMed] [Google Scholar]
- 20.Foti G, Sangalli F, Berra L, Sironi S, Cazzaniga M, Rossi GP, et al. Is helmet CPAP first line pre-hospital treatment of presumed severe acute pulmonary edema? Intens Care Med. 2009;35:656–62. [DOI] [PubMed] [Google Scholar]
- 21.Antonelli M, Pennisi MA, Pelosi P, Gregoretti C, Squadrone V, Rocco M, et al. Noninvasive positive pressure ventilation using a helmet in patients with acute exacerbation of chronic obstructive pulmonary disease. Anesthesiology. 2004;100:16–24. [DOI] [PubMed] [Google Scholar]
- 22.Antonaglia V, Ferluga M, Molino R, Lucangelo U, Peratoner A, Roman-Pognuz E, et al. Comparison of noninvasive ventilation by sequential use of mask and helmet versus mask in acute exacerbation of chronic obstructive pulmonary disease: a preliminary study. Respiration. 2011;82:148–54. [DOI] [PubMed] [Google Scholar]
- 23.Plotnikow GA, Accoce M, Fredes S, Tiribelli N, Setten M, Dorado J, et al. High-Flow oxygen therapy application in chronic obstructive pulmonary disease patients with acute hypercapnic respiratory failure: a multicenter study. Crit Care Explor. 2021;3:e0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Shan M, Zhu H, Cao J, Chen R. Noninvasive ventilation with a helmet in patients with acute respiratory failure caused by chest trauma: a randomized controlled trial. Sci Rep. 2020;10:21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Q, Tan D, Wang H, Zhao R, Ling B. High-flow nasal cannula oxygen therapy for mild-moderate acute respiratory failure in patients with blunt chest trauma: an exploratory descriptive study. Am J Emerg Med. 2024;83:76–81. [DOI] [PubMed] [Google Scholar]
- 26.Principi T, Pantanetti S, Catani F, Elisei D, Gabbanelli V, Pelaia P, et al. Noninvasive continuous positive airway pressure delivered by helmet in hematological malignancy patients with hypoxemic acute respiratory failure. Intens Care Med. 2004;30:147–50. [DOI] [PubMed] [Google Scholar]
- 27.Rabitsch W, Schellongowski P, Köstler WJ, Stoiser B, Knöbl P, Locker GJ, et al. Efficacy and tolerability of non-invasive ventilation delivered via a newly developed helmet in immunosuppressed patients with acute respiratory failure. Wien Klin Wochenschr. 2003;115:590. [DOI] [PubMed] [Google Scholar]
- 28.Rocco M, Dell’Utri D, Morelli A, Spadetta G, Conti G, Antonelli M, et al. Noninvasive ventilation by helmet or face mask in immunocompromised patients. Chest. 2004;126:1508–15. [DOI] [PubMed] [Google Scholar]
- 29.Kang H, Zhao Z, Tong Z. Effect of high-flow nasal cannula oxygen therapy in immunocompromised subjects with acute respiratory failure. Respir Care. 2020;65:369–76. [DOI] [PubMed] [Google Scholar]
- 30.Carrillo A, Gonzalez-Diaz G, Ferrer M, Martinez-Quintana ME, Lopez-Martinez A, Llamas N, et al. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intens Care Med. 2012;38:458–66. [DOI] [PubMed] [Google Scholar]
- 31.Bellani G, Laffey JG, Pham T, Madotto F, Fan E, Brochard L, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome: insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195:67–77. [DOI] [PubMed] [Google Scholar]
- 32.Antonelli M, Conti G, Moro M, Esquinas A, Gonzalez-Diaz G, Confalonieri M, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intens Care Med. 2001;27:1718–28. [DOI] [PubMed] [Google Scholar]
- 33.Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199:1368–76. [DOI] [PubMed] [Google Scholar]
- 34.Thille AW, Contou D, Fragnoli C, Córdoba-Izquierdo A, Boissier F, Brun-Buisson C. Non-invasive ventilation for acute hypoxemic respiratory failure: intubation rate and risk factors. Crit Care. 2013. 10.1186/cc13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demoule A, Chevret S, Carlucci A, Kouatchet A, Jaber S, Meziani F, et al. Changing use of noninvasive ventilation in critically ill patients: trends over 15 years in francophone countries. Intens Care Med. 2016;42:82–92. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Duan J, Zhou L. Incidence of noninvasive ventilation failure and mortality in patients with acute respiratory distress syndrome: a systematic review and proportion meta-analysis. BMC Pulm Med. 2024. 10.1186/s12890-024-02839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain SNA, Roussos C. Distribution of respiratory muscle and organ blood flow during endotoxic shock in dogs. J Appl Physiol. 1985;59:1802–8. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med. 2013;41:536–45. [DOI] [PubMed] [Google Scholar]
- 39.Slobod D, Spinelli E, Crotti S, Lissoni A, Galazzi A, Grasselli G, et al. Effects of an asymmetrical high flow nasal cannula interface in hypoxemic patients. Crit Care. 2023. 10.1186/s13054-023-04441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauri T, Alban L, Turrini C, Cambiaghi B, Carlesso E, Taccone P, et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intens Care Med. 2017;43:1453–63. [DOI] [PubMed] [Google Scholar]
- 41.Pinkham M, Tatkov S. Effect of flow and cannula size on generated pressure during nasal high flow. Crit Care. 2020;24:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatkov S, Rees M, Gulley A, van den Heuij LGT, Nilius G. Asymmetrical nasal high flow ventilation improves clearance of CO2 from the anatomical dead space and increases positive airway pressure. J Appl Physiol. 1985;2023(134):365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coppadoro A, Bellani G, Foti G. A technique to measure tidal volume during noninvasive respiratory support by continuous-flow helmet CPAP. J Clin Monit Comput. 2023;37:1473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lellouche F, L’Her E, Abroug F, Deye N, Rodriguez PO, Rabbat A, et al. Impact of the humidification device on intubation rate during noninvasive ventilation with ICU ventilators: results of a multicenter randomized controlled trial. Intens Care Med. 2014;40:211–9. [DOI] [PubMed] [Google Scholar]
- 45.Ferreyro BL, De Jong A, Grieco DL. How to use facemask noninvasive ventilation. Intens Care Med. 2024;50:1346–9. [DOI] [PubMed] [Google Scholar]
- 46.Rosà T, Menga LS, Tejpal A, Cesarano M, Michi T, Sklar MC, et al. Non-invasive ventilation for acute hypoxemic respiratory failure, including COVID-19. J Intens Med. 2023;3:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cesarano M, Grieco DL, Michi T, Munshi L, Menga LS, Delle Cese L, et al. Helmet noninvasive support for acute hypoxemic respiratory failure: rationale, mechanism of action and bedside application. Ann Intens Care. 2022;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grieco DL, Maggiore SM, Roca O, Spinelli E, Patel BK, Thille AW, et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intens Care Med. 2021;47:851–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yarnell CJ, Johnson A, Dam T, Jonkman A, Liu K, Wunsch H, et al. Do thresholds for invasive ventilation in hypoxemic respiratory failure exist? A cohort study. Am J Respir Crit Care Med. 2023;207:271–82. [DOI] [PubMed] [Google Scholar]
- 50.Ajmani GS, Patel BK. To intubate or not intubate, that is the question. Am J Respir Crit Care Med. 2023;207:233–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–42. [DOI] [PubMed] [Google Scholar]
- 52.Cruces P, Erranz B, Pérez A, Reveco S, González C, Retamal J, et al. Noninvasive continuous positive airway pressure is a lung- and diaphragm-protective approach in self-inflicted lung injury. Am J Respir Crit Care Med. 2024;209:1022–5. [DOI] [PubMed] [Google Scholar]
- 53.Jiang T-X, Reid WD, Belcastro A, Road JD. Load dependence of secondary diaphragm inflammation and injury after acute inspiratory loading. Am J Respir Crit Care Med. 1998;157:230–6. [DOI] [PubMed] [Google Scholar]
- 54.Goligher EC, Brochard LJ, Reid WD, Fan E, Saarela O, Slutsky AS, et al. Diaphragmatic myotrauma: a mediator of prolonged ventilation and poor patient outcomes in acute respiratory failure. Lancet Respir Med. 2019;7:90–8. [DOI] [PubMed] [Google Scholar]
- 55.Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–31. [DOI] [PubMed] [Google Scholar]
- 56.Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Executive summary: clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:1532–48. [DOI] [PubMed] [Google Scholar]
- 57.Slutsky AS, Ranieri VM. Ventilator-Induced Lung Injury. N Engl J Med. 2013;369:2126–36. [DOI] [PubMed] [Google Scholar]
- 58.Le Marec J, Hajage D, Decavèle M, Schmidt M, Laurent I, Ricard J-D, et al. High airway occlusion pressure is associated with dyspnea and increased mortality in critically Ill mechanically ventilated patients. Am J Respir Crit Care Med. 2024;210:201–10. [DOI] [PubMed] [Google Scholar]
- 59.Pérez J, Telias I. Airway Occlusion Pressure and Dyspnea during Mechanical Ventilation: Giving Words to the Pleas of the Respiratory Centers. Am J Respir Crit Care Med. American Thoracic Society; 2024. p. 139–41. [DOI] [PMC free article] [PubMed]
- 60.Dangers L, Montlahuc C, Kouatchet A, Jaber S, Meziani F, Perbet S, et al. Dyspnoea in patients receiving noninvasive ventilation for acute respiratory failure: Prevalence, risk factors and prognostic impact. European Respiratory Journal. 2018;52. [DOI] [PubMed]
- 61.Demoule A, Baptiste A, Thille AW, Similowski T, Ragot S, Prat G, et al. Dyspnea is severe and associated with a higher intubation rate in de novo acute hypoxemic respiratory failure. Crit Care. 2024. 10.1186/s13054-024-04903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Decavele M, Demoule A, Similowski T. Detection and management of dyspnea In mechanically ventilated patients. Curr Opin Crit Care. 2019;25:86–94. [DOI] [PubMed] [Google Scholar]
- 63.Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume∗. Crit Care Med. 2016;44:282–90. [DOI] [PubMed] [Google Scholar]
- 64.Frat JP, Ragot S, Coudroy R, Constantin JM, Girault C, Prat G, et al. Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a noninvasive oxygenation strategy. Critic Care Med. 2018;46:208–15. [DOI] [PubMed] [Google Scholar]
- 65.Tonelli R, Fantini R, Tabbì L, Castaniere I, Pisani L, Pellegrino MR, et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de novo respiratory failure: a pilot study. Am J Respir Crit Care Med. 2020;202:558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haudebourg AF, Maraffi T, Tuffet S, Le Corvoisier P, Mekontso Dessap A, Carteaux G. Influence of different noninvasive oxygenation support devices on tidal volume. Ann Intens Care. 2023. 10.1186/s13613-023-01200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Docci M, Foti G, Brochard L, Bellani G. Pressure support, patient effort and tidal volume: a conceptual model for a non linear interaction. Crit Care. 2024;28:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo Z, Li Y, Li W, Li Y, Nie Q, Shi Y, et al. Effect of high-intensity vs low-intensity noninvasive positive pressure ventilation on the need for endotracheal intubation in patients with an acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2024;332:1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thille AW, Cabello B, Galia F, Lyazidi A, Brochard L. Reduction of patient-ventilator asynchrony by reducing tidal volume during pressure-support ventilation. Intens Care Med. 2008;34:1477–86. [DOI] [PubMed] [Google Scholar]
- 70.Luján M, Lalmolda C. Ventilators, settings, autotitration algorithms. J Clin Med. 2023;12:2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luján M, Lalmolda C, Ergan B. Basic concepts for tidal volume and leakage estimation in non-invasive ventilation. Turk Thorac J. 2019;20:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luján M, Sogo A, Grimau C, Pomares X, Blanch L, Monsó E. Influence of dynamic leaks in volume-targeted pressure support noninvasive ventilation: a bench study. Respir Care. 2015;60:191–200. [DOI] [PubMed] [Google Scholar]
- 73.Cortegiani A, Navalesi P, Accurso G, Sabella I, Misseri G, Ippolito M, et al. Tidal volume estimation during helmet noninvasive ventilation: an experimental feasibility study. Sci Rep. 2019. 10.1038/s41598-019-54020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Z, Gao Y, Zhao Z. Volume-OXygenation index to predict high-flow nasal cannula failure: how to capture the tidal volume matters. Am J Respir Crit Care Med. 2023;207:490–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gatti S, Rezoagli E, Madotto F, Foti G, Bellani G. A non-invasive continuous and real-time volumetric monitoring in spontaneous breathing subjects based on bioimpedance—ExSpiron®Xi: a validation study in healthy volunteers. J Clin Monit Comput. 2024;38:539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically Ill patients pathophysiology and clinical implications. Am J Respir Crit Care Med. 2020;201:20–32. [DOI] [PubMed] [Google Scholar]
- 77.Akoumianaki E, Vaporidi K, Georgopoulos D. The injurious effects of elevated or nonelevated respiratory rate during mechanical ventilation. Am J Respir Crit Care Med. 2019;199:149–57. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida Y, Takeda S, Akada S, Hongo T, Tanaka K, Sakamoto A. Factors predicting successful noninvasive ventilation in acute lung injury. J Anesth. 2008;22:201–6. [DOI] [PubMed] [Google Scholar]
- 79.Duan J, Han X, Bai L, Zhou L, Huang S. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intens Care Med. 2017;43:192–9. [DOI] [PubMed] [Google Scholar]
- 80.Praphruetkit N, Boonchana N, Monsomboon A, Ruangsomboon O. ROX index versus HACOR scale in predicting success and failure of high-flow nasal cannula in the emergency department for patients with acute hypoxemic respiratory failure: a prospective observational study. Int J Emerg Med. 2023. 10.1186/s12245-023-00477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coppadoro A, Benini A, Fruscio R, Verga L, Mazzola P, Bellelli G, et al. Helmet CPAP to treat hypoxic pneumonia outside the ICU: an observational study during the COVID-19 outbreak. Crit Care. 2021;25:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He H, Sun B, Liang L, Li Y, Wang H, Wei L, et al. A multicenter RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome. Crit Care. 2019;23:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jolliet P, Abajo B, Pasquina P, Chevrolet JC. Non-invasive pressure support ventilation in severe community-acquired pneumonia. Intens Care Med. 2001;27:812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cei F, Chiarugi L, Brancati S, Dolenti S, Montini MS, Rosselli M, et al. Clinical and personal predictors of helmet-CPAP use and failure in patients firstly admitted to regular medical wards with COVID-19-related acute respiratory distress syndrome (hCPAP-f study). Biomedicines. 2023;11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen D, Heunks L, Pan C, Xie J, Qiu H, Yang Y, et al. A novel index to predict the failure of high-flow nasal cannula in patients with acute hypoxemic respiratory failure: a pilot study. Am J Respir Crit Care Med. 2022;206:910–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.León-Valladares D, Barrio-Mateu LA, Cortés-Carmona N, Fuentes-Lizana G, Cabanas AM, Latorre-Progulakis K, et al. Determining factors of pulse oximetry accuracy: a literature review. Rev Clin Esp. 2024;224:314–30. [DOI] [PubMed] [Google Scholar]
- 87.Jacono FJ, Peng Y-J, Nethery D, Faress JA, Lee Z, Kern JA, et al. HIGHLIGHTED TOPIC reflexes from the lungs and airways acute lung injury augments hypoxic ventilatory response in the absence of systemic hypoxemia. J Appl Physiol. 2006;101:1795–802. [DOI] [PubMed] [Google Scholar]
- 88.Telias I, Spadaro S. Techniques to monitor respiratory drive and inspiratory effort. Curr Opin Crit Care. 2020;26:3–10. [DOI] [PubMed] [Google Scholar]
- 89.Consalvo S, Accoce M, Telias I. Monitoring and modulating respiratory drive in mechanically ventilated patients. Curr Opin Crit Care. 2024. 10.1097/MCC.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 90.trenchard1972—Vgal afferences mediated RSB pattern.
- 91.Xu X, Ma M, Min Y, Hu W, Bai L, Duan J. PaCO2 is nonlinearly associated with NIV failure in patients with hypoxemic respiratory failure. BMC Pulm Med. 2024. 10.1186/s12890-024-03023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luca Grieco D, Menga L, Cesarano M, Spadaro S, Maddalena Bitondo M, Berardi C, et al. Phenotypes of patients with COVID-19 who have a positive clinical response to helmet noninvasive ventilation. Am J Respir Crit Care Med. 2022;205:360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perkhofer L, Strobel A, Gagiannis D, Seufferlein T, Schmidt K, Mayer B, et al. Transcutaneous carbon dioxide monitoring as a valid complementary method in acute respiratory failure. Eur Respir J. 2020;56:2002137. [DOI] [PubMed] [Google Scholar]
- 94.Jonkman AH, Telias I, Spinelli E, Akoumianaki E, Piquilloud L. The oesophageal balloon for respiratory monitoring in ventilated patients: updated clinical review and practical aspects. Eur Respir Rev. 2023;32:220186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–31. [DOI] [PubMed] [Google Scholar]
- 96.Baydur A, Cha E-J, Sassoon CSH Validation of esophageal balloon technique t different lung volumes and postures [Internet]. 1987. Available from: www.physiology.org/journal/jappl [DOI] [PubMed]
- 97.Mercurio G, D’Arrigo S, Moroni R, Grieco DL, Menga LS, Romano A, et al. Diaphragm thickening fraction predicts noninvasive ventilation outcome: a preliminary physiological study. Crit Care. 2021. 10.1186/s13054-021-03638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Colombo J, Spinelli E, Grasselli G, Pesenti AM, Protti A. Detection of strong inspiratory efforts from the analysis of central venous pressure swings: a preliminary clinical study. Minerva Anestesiol. 2020;86:1296–304. [DOI] [PubMed] [Google Scholar]
- 99.Lassola S, Miori S, Sanna A, Menegoni I, De Rosa S, Bellani G, et al. Assessment of inspiratory effort in spontaneously breathing COVID-19 ARDS patients undergoing helmet CPAP: a comparison between esophageal, transdiaphragmatic and central venous pressure swing. Diagnostics. 2023;13:1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tonelli R, Cortegiani A, Marchioni A, Fantini R, Tabbì L, Castaniere I, et al. Nasal pressure swings as the measure of inspiratory effort in spontaneously breathing patients with de novo acute respiratory failure. Critic Care. 2022. 10.1186/s13054-022-03938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tonelli R, Cortegiani A, Fantini R, Tabbì L, Castaniere I, Bruzzi G, et al. Accuracy of nasal pressure swing to predict failure of high-flow nasal oxygen in patients with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2023;207:787–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pérez J, Dorado JH, Papazian AC, Berastegui M, Gilgado DI, Cardoso GP, et al. Titration and characteristics of pressure-support ventilation use in Argentina: an online cross-sectional survey study. Rev Bras Ter Intensiva. 2020;32:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hudson AL, Gandevia SC, Butler JE. The effect of lung volume on the co-ordinated recruitment of scalene and sternomastoid muscles in humans. J Physiol. 2007;584:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bellemare F, Grassino A. Effect of pressure and timing of contraction on human diaphragm fatigue ’f* [Internet]. 1982. Available from: www.physiology.org/journal/jappl [DOI] [PubMed]
- 105.Roesthuis LH, van der Hoeven JG, van Hees HWH, Schellekens WJM, Doorduin J, Heunks LMA. Recruitment pattern of the diaphragm and extradiaphragmatic inspiratory muscles in response to different levels of pressure support. Ann Intens Care. 2020. 10.1186/s13613-020-00684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Santus P, Pini S, Amati F, Saad M, Gatti M, Mondoni M, et al. Predictors of helmet CPAP failure in COVID-19 pneumonia: a prospective, multicenter, and observational cohort study. Can Respir J. 2022;2022:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duan J, Chen L, Liu X, Bozbay S, Liu Y, Wang K, et al. An updated HACOR score for predicting the failure of noninvasive ventilation: a multicenter prospective observational study. Crit Care. 2022. 10.1186/s13054-022-04060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Colaianni-Alfonso N, Montiel GC, Castro-Sayat M, Roca O, Grieco DL. ROX index to predict CPAP outcome in hypoxemic respiratory failure due to COVID-19. Intens Care Med. 2022;48:1818–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duan J, Yang J, Jiang L, Bai L, Hu W, Shu W, et al. Prediction of noninvasive ventilation failure using the ROX index in patients with de novo acute respiratory failure. Ann Intens Care. 2022. 10.1186/s13613-022-01085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Protti A, Tonelli R, Dalla Corte F, Grieco DL, Spinelli E, Spadaro S, et al. Development of clinical tools to estimate the breathing effort during high-flow oxygen therapy: a multicenter cohort study. Pulmonology. 2024. 10.1016/j.pulmoe.2024.04.008. [DOI] [PubMed] [Google Scholar]
- 111.Biasucci DG, Buonsenso D, Piano A, Bonadia N, Vargas J, Settanni D, et al. Lung ultrasound predicts non-invasive ventilation outcome in COVID-19 acute respiratory failure: a pilot study. Minerva Anestesiol. 2021;87:1006–16. [DOI] [PubMed] [Google Scholar]
- 112.Nova A, Rezoagli E, Eronia N, Benini A, Scognamiglio A, Foti G, et al. Prognostic performance of bedside lung ultrasound score (LUSS) and ROX index in hypoxemic respiratory failure due to COVID-19. Diagnostics. 2023;13:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scaramuzzo G, Pavlovsky B, Adler A, Baccinelli W, Bodor DL, Damiani LF, et al. Electrical impedance tomography monitoring in adult ICU patients: state-of-the-art, recommendations for standardized acquisition, processing, and clinical use, and future directions. Crit Care. 2024;25:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao Z, Chang MY, Zhang T, Gow CH. Monitoring the efficacy of high-flow nasal cannula oxygen therapy in patients with acute hypoxemic respiratory failure in the general respiratory ward: a prospective observational study. Biomedicines. 2023;11:3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu L, Wang X, Hu P, Pan Y, Zhao N, Lu Y, et al. Monitoring the Pendelluft by EIT could predict the failure of non-invasive mechanical ventilation: a prospective study [Internet]. 2024. Available from: https://www.researchsquare.com/article/rs-4315149/v1
- 116.Kang BJ, Koh Y, Lim CM, Huh JW, Baek S, Han M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intens Care Med. 2015;41:623–32. [DOI] [PubMed] [Google Scholar]
- 117.Dumas G, Lemiale V, Rathi N, Cortegiani A, Pené F, Bonny V, et al. Survival in immunocompromised patients ultimately requiring invasive mechanical ventilation: a pooled individual patient data analysis. Am J Respir Crit Care Med. 2021;204:187–96. [DOI] [PubMed] [Google Scholar]
- 118.Mellado-Artigas R, Borrat X, Ferreyro BL, Yarnell C, Hao S, Wanis KN, et al. Effect of immediate initiation of invasive ventilation on mortality in acute hypoxemic respiratory failure: a target trial emulation. Crit Care. 2024;28:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yarnell CJ, Paranthaman A, Reardon P, Angriman F, Bassi T, Bellani G, et al. An international factorial vignette-based survey of intubation decisions in acute hypoxemic respiratory failure. Crit Care Med. 2024;53:e117–31. [DOI] [PubMed] [Google Scholar]
- 120.https://practicalplatform.org.
- 121.Telias I, Brochard L, Goligher EC. Is my patient’s respiratory drive (too) high? Intens Care Med. 2018;44:1936–9. [DOI] [PubMed] [Google Scholar]
- 122.Bertoni M, Telias I, Urner M, Long M, Del Sorbo L, Fan E, et al. A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care. 2019. 10.1186/s13054-019-2617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gogniat E, Steinberg E, Tiribelli N, Setten M, Gutierrez FJ, Plotnikow GA. Validation of airway occlusion pressure as a method of assessing breathing effort during noninvasive ventilation. Respir Care. 2025. 10.1089/respcare.12324. [DOI] [PubMed] [Google Scholar]
- 124.Dargent A, Hombreux A, Roccia H, Argaud L, Cour M, Guérin C. Feasibility of non-invasive respiratory drive and breathing pattern evaluation using CPAP in COVID-19 patients. J Crit Care. 2022;69:154020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hilbert G, Gruson D, Portel L, Vargas F, Gbikpi-Benissan G, Cardinaud JP. Airway occlusion pressure at 0.1 s (P0.1) after extubation: an early indicator of postextubation hypercapnic respiratory insufficiency. Intensive Care Med. 1998;24:1277–82. [DOI] [PubMed] [Google Scholar]
- 126.Sosio S, Bellani G, Villa S, Lupieri E, Mauri T, Foti G. A Calibration technique for the estimation of lung volumes in nonintubated subjects by electrical impedance tomography. Respiration. 2019;98:189–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.