Abstract
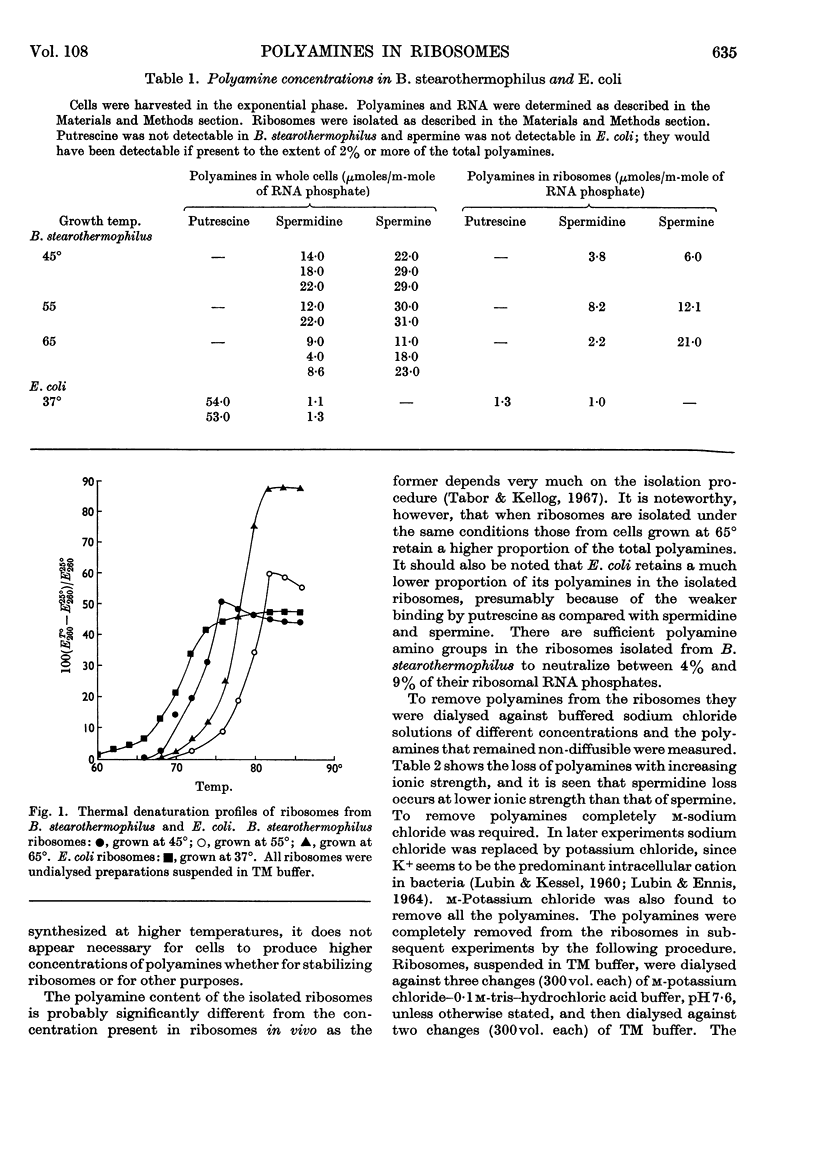
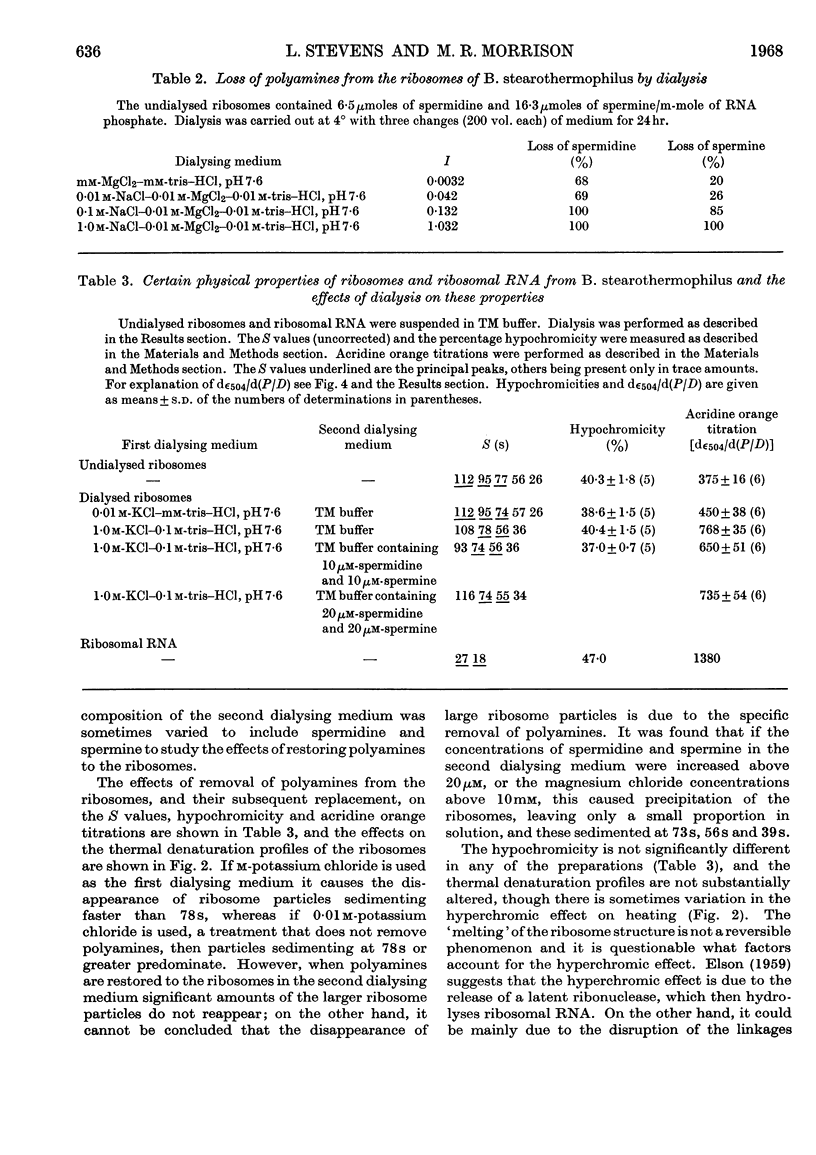
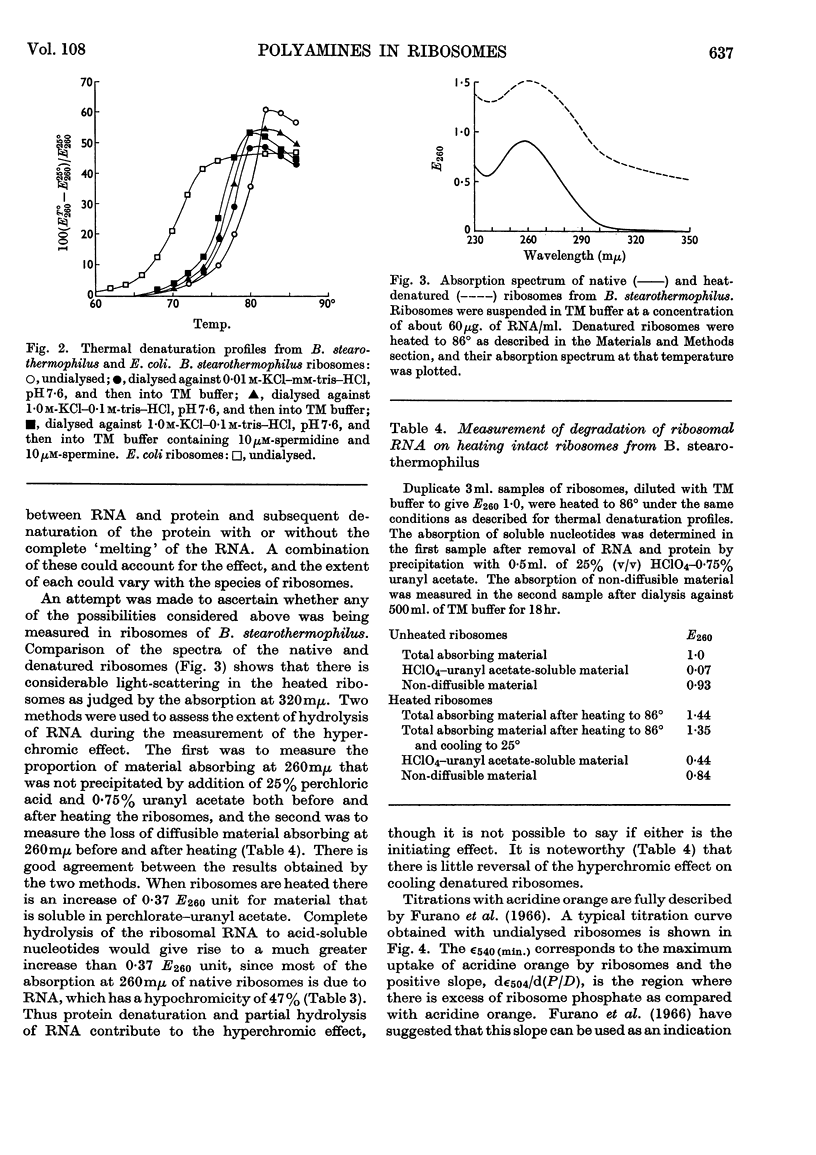
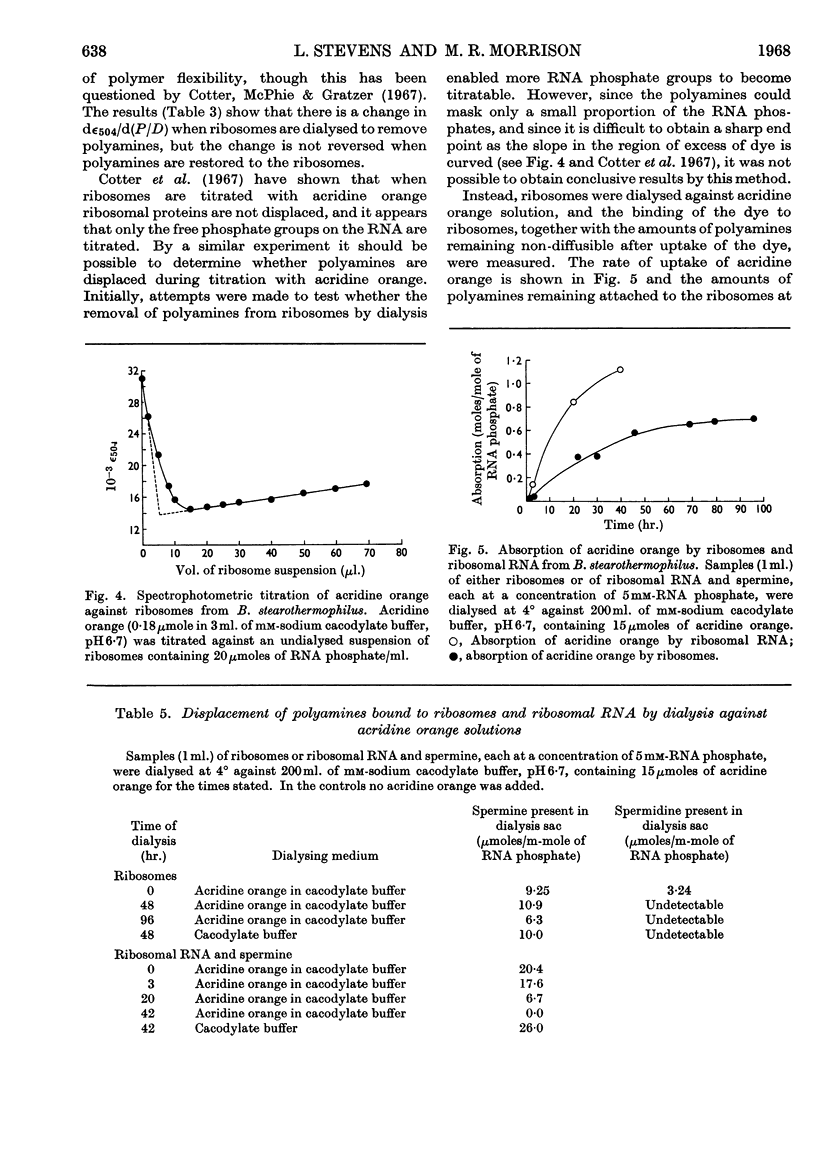
1. Spermine and spermidine were the main polyamines detectable in Bacillus stearothermophilus. 2. When grown at 65° B. stearothermophilus contained lower concentrations of polyamines per mg. of RNA than when grown at 45° or at 55°. 3. Ribosomes isolated from B. stearothermophilus in 0·01m-tris–hydrochloric acid buffer (pH7·4)–0·01m-magnesium chloride contained sufficient polyamines to neutralize between 4% and 9% of their RNA phosphorus. 4. Removal of polyamines from the ribosomes by dialysis against m-potassium chloride did not appreciably alter the hypochromicity or thermal denaturation profiles of the ribosomes when measured in 0·01m-tris–hydrochloric acid buffer (pH7·4)–0·01m-magnesium chloride, though it did cause a loss of ribosome particles sedimenting at greater than 78s. 5. When ribosomes were dialysed against acridine orange solutions acridine orange bound to the ribosomes and did not displace spermine, but when a mixture of ribosomal RNA and spermine was dialysed against acridine orange the acridine orange displaced the spermine. It is concluded that polyamines in the ribosomes are less accessible for displacement by acridine orange than when polyamines are bound to ribosomal RNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BACHRACH U., COHEN I. Spermidine in the bacterial cell. J Gen Microbiol. 1961 Sep;26:1–9. doi: 10.1099/00221287-26-1-1. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- COHEN S. S., LICHTENSTEIN J. Polyamines and ribosome structure. J Biol Chem. 1960 Jul;235:2112–2116. [PubMed] [Google Scholar]
- Cotter R. I., McPhie P., Gratzer W. B. Internal organization of the ribosome. Nature. 1967 Dec 2;216(5118):864–868. doi: 10.1038/216864a0. [DOI] [PubMed] [Google Scholar]
- ELSON D. Latent enzymic activity of a ribonucleoprotein isolated from Escherichia coli. Biochim Biophys Acta. 1959 Dec;36:372–386. doi: 10.1016/0006-3002(59)90179-9. [DOI] [PubMed] [Google Scholar]
- Friedman S. M., Axel R., Weinstein I. B. Stability of ribosomes and ribosomal ribonucleic acid from Bacillus stearothermophilus. J Bacteriol. 1967 May;93(5):1521–1526. doi: 10.1128/jb.93.5.1521-1526.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V., Bradley D. F., Childers L. G. The conformation of the ribonucleic acid in ribosomes. Dye stacking studies. Biochemistry. 1966 Sep;5(9):3044–3056. doi: 10.1021/bi00873a038. [DOI] [PubMed] [Google Scholar]
- HERBST E. J., WEAVER R. H., KEISTER D. L. The gram reaction and cell composition: diamines and polyamines. Arch Biochem Biophys. 1958 May;75(1):171–177. doi: 10.1016/0003-9861(58)90407-7. [DOI] [PubMed] [Google Scholar]
- LUBIN M., ENNIS H. L. ON THE ROLE OF INTRACELLULAR POTASSIUM IN PROTEIN SYNTHESIS. Biochim Biophys Acta. 1964 Apr 27;80:614–631. doi: 10.1016/0926-6550(64)90306-8. [DOI] [PubMed] [Google Scholar]
- MOLLER M. L., KIM K. EFFECTS OF PUTRESCINE AND MAGNESIUM ON THE RIBOSOMES OF A PSEUDOMONAS. Biochem Biophys Res Commun. 1965 Jun 18;20:46–52. doi: 10.1016/0006-291x(65)90948-4. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Mangiantini M. T., Tecce G., Toschi G., Trentalance A. A study of ribosomes and of ribonucleic acid from a thermorphilic organism. Biochim Biophys Acta. 1965 Jun 8;103(2):252–274. doi: 10.1016/0005-2787(65)90166-8. [DOI] [PubMed] [Google Scholar]
- Pace B., Campbell L. L. Correlation of maximal growth temperature and ribosome heat stability. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1110–1116. doi: 10.1073/pnas.57.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Cohen S. S. Polyamines and RNA synthesis in a polyauxotrophic strain of E. coli. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1587–1593. doi: 10.1073/pnas.55.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders G. F., Campbell L. L. Ribonucleic acid and ribosomes of Bacillus stearothermophilus. J Bacteriol. 1966 Jan;91(1):332–339. doi: 10.1128/jb.91.1.332-339.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silman N., Artman M., Engleberg H. Effect of magnesium and spermine on the aggregation of bacterial and mammalian ribosomes. Biochim Biophys Acta. 1965 Jun 8;103(2):231–240. doi: 10.1016/0005-2787(65)90164-4. [DOI] [PubMed] [Google Scholar]
- Stevens L. Studies on the interaction of homologues of spermine with deoxyribonucleic acid and with bacterial protoplasts. Biochem J. 1967 Jun;103(3):811–815. doi: 10.1042/bj1030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- TABOR H. The protective effect of spermine and other polyamines against heat denaturation of deoxyribonucleic acid. Biochemistry. 1962 May 25;1:496–501. doi: 10.1021/bi00909a021. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Kellogg P. D. The effect of isolation conditions on the polyamine content of Escherichia coli ribosomes. J Biol Chem. 1967 Mar 10;242(5):1044–1052. [PubMed] [Google Scholar]


