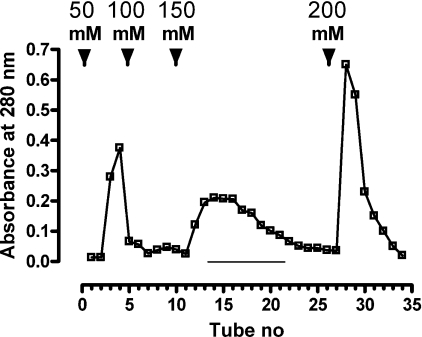
Figure 1. Purification of chLBD extracted from IBs and refolded on a Q-Sepharose column.
The column (2.5 cm×7 cm) was equilibrated with 10 mM Tris/HCl (pH 9.0) at 4 °C. The dialysed solution of refolded protein was applied to the column at a rate of 400 ml/h. Elution was carried out using a discontinuous NaCl gradient in the same buffer at 400 ml/h, and 50 ml fractions were collected. Protein concentration was determined by measuring absorbance at 280 nm. Every third tube was analysed for chLBD content by gel filtration in a Superdex™ 75 HR column. Fractions eluted with 150 mM NaCl (underlined) contained monomeric protein and were pooled.