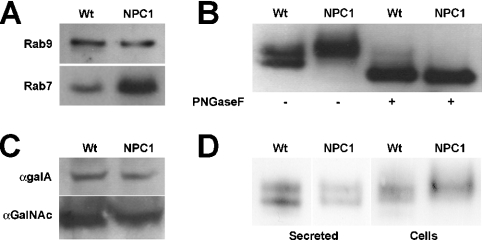
Figure 4. Analysis of proteins associated with Wt and NPC1 purified late endosomes.
(A) Association of Rab7, but not Rab9, is altered in NPC1 endosomes. Protein (5 μg) from Wt and NPC1 endosomes was subjected to electrophoresis and Western blotting against Rab7 and Rab9. Following quantification, the amount of Rab7 in NPC1 late endosomes was found to be approx. 3-fold higher than the amount found in Wt late endosomes. (B) Microheterogeneity of NPC2 in NPC1 vesicles is due to variations in N-linked oligosaccharides. Protein (10 μg) from Wt and NPC1 vesicles was incubated with (+) or without (−) PNGase F and then subjected to electrophoresis and Western blotting against NPC2. (C) Processing of soluble lysosomal enzymes is normal in NPC1 vesicles. Protein (10 μg) from Wt and NPC1 vesicles was subjected to electrophoresis and Western blotting against α-galactosidase A and α-N-acetylgalactosaminidase. (D) NPC2 protein destined for the secretory pathway in NPC1 mice does not show the same altered processing as the endosome-associated NPC2 protein. Cells derived from Wt and NPC1 mice were cultured for 3 days in serum-free medium, after which an amount of medium equivalent to 3 μg of total cellular protein was subjected to electrophoresis and Western blotting against NPC2. The secreted NPC2 protein in the medium (secreted) from Wt and NPC1 cells was identical, in contrast with the cellular NPC2 (cells) detected in 10 μg of cell lysate.