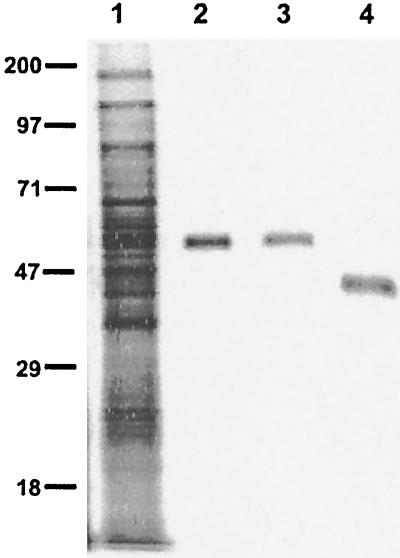
FIG. 1.
Purified i-antigen of I. multifiliis G5 isolate. The G5 i-antigen was purified by immunoaffinity chromatography with immobilizing MAb G3-61 coupled to an AminoLink matrix. The purified i-antigen was resolved by SDS-PAGE on a 10% polyacrylamide gel and stained with silver nitrate. Lane 1, total membrane proteins of I. multifiliis G5 isolate (10 μg); lanes 2 and 3, reduced purified G5 i-antigen (0.5 μg); lane 4, nonreduced purified G5 i-antigen (0.5 μg). Protein standards (molecular masses [in kilodaltons]) are indicated to the left. The molecular mass of the reduced G5 i-antigen is ≈55 kDa, and that of the nonreduced G5 i-antigen is ≈46 kDa.