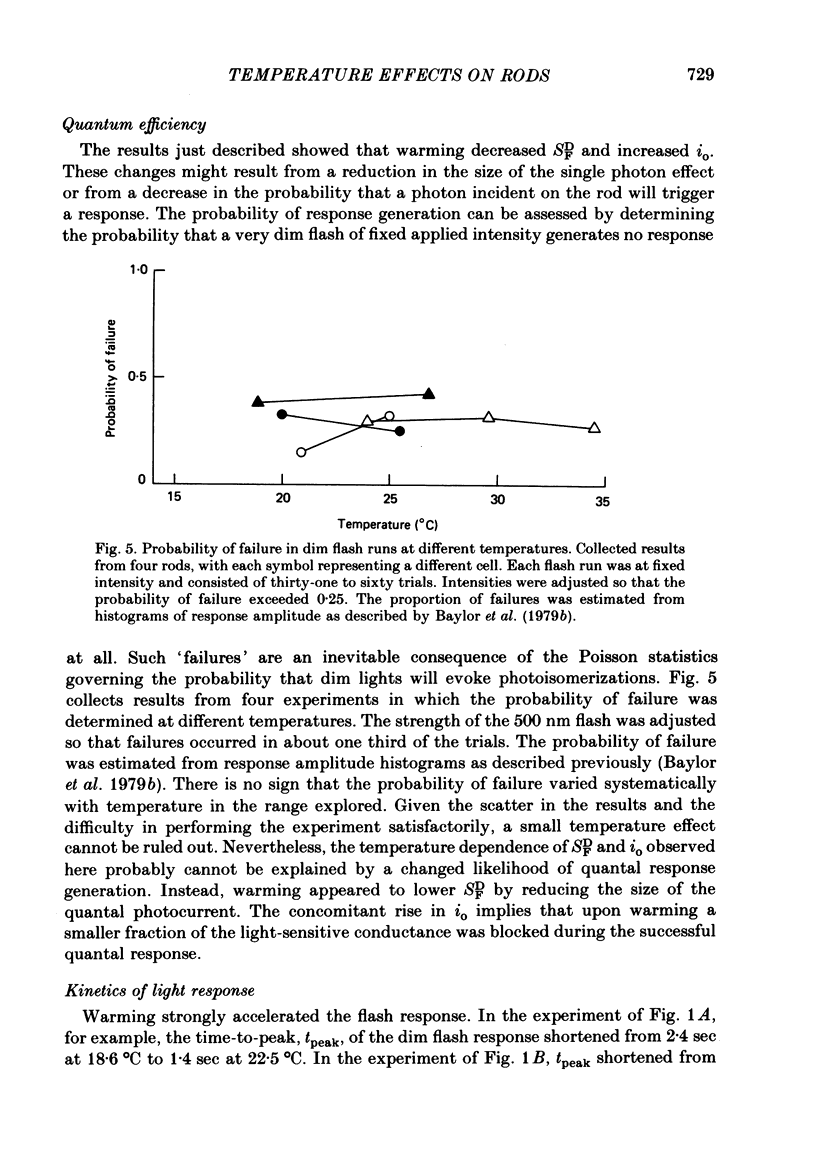
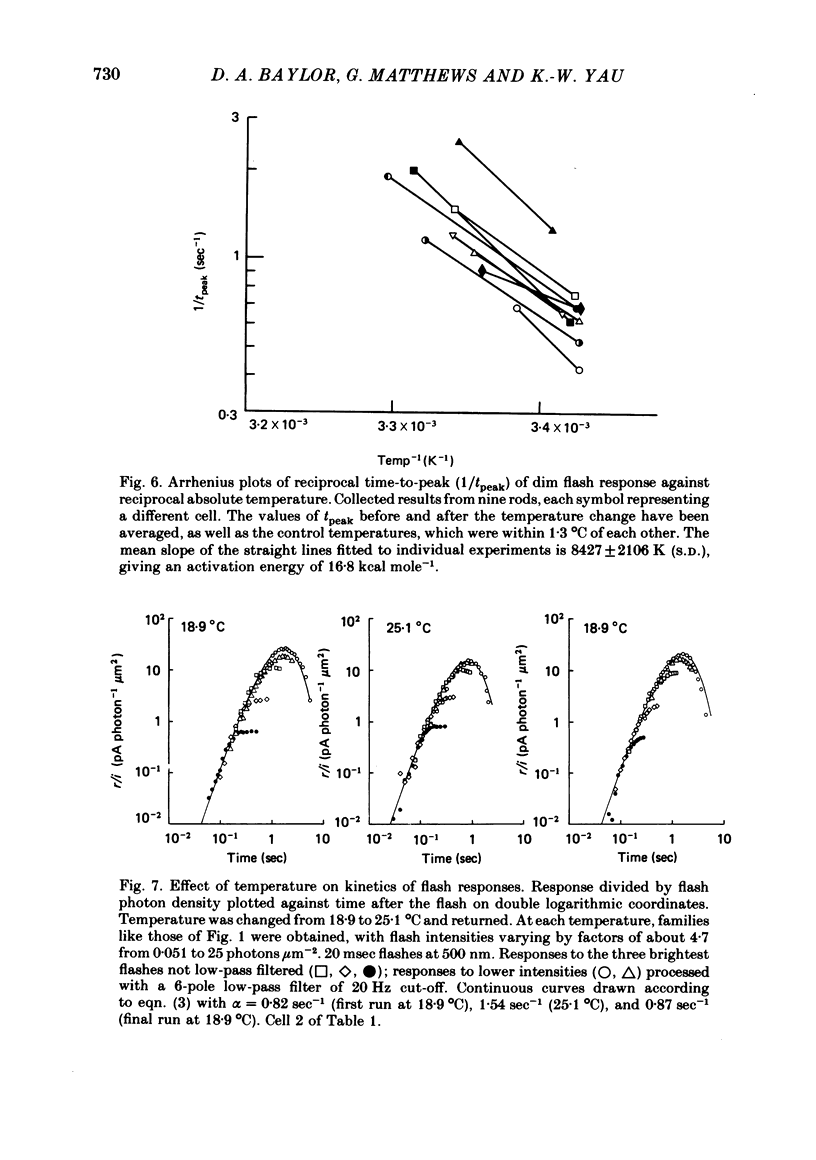
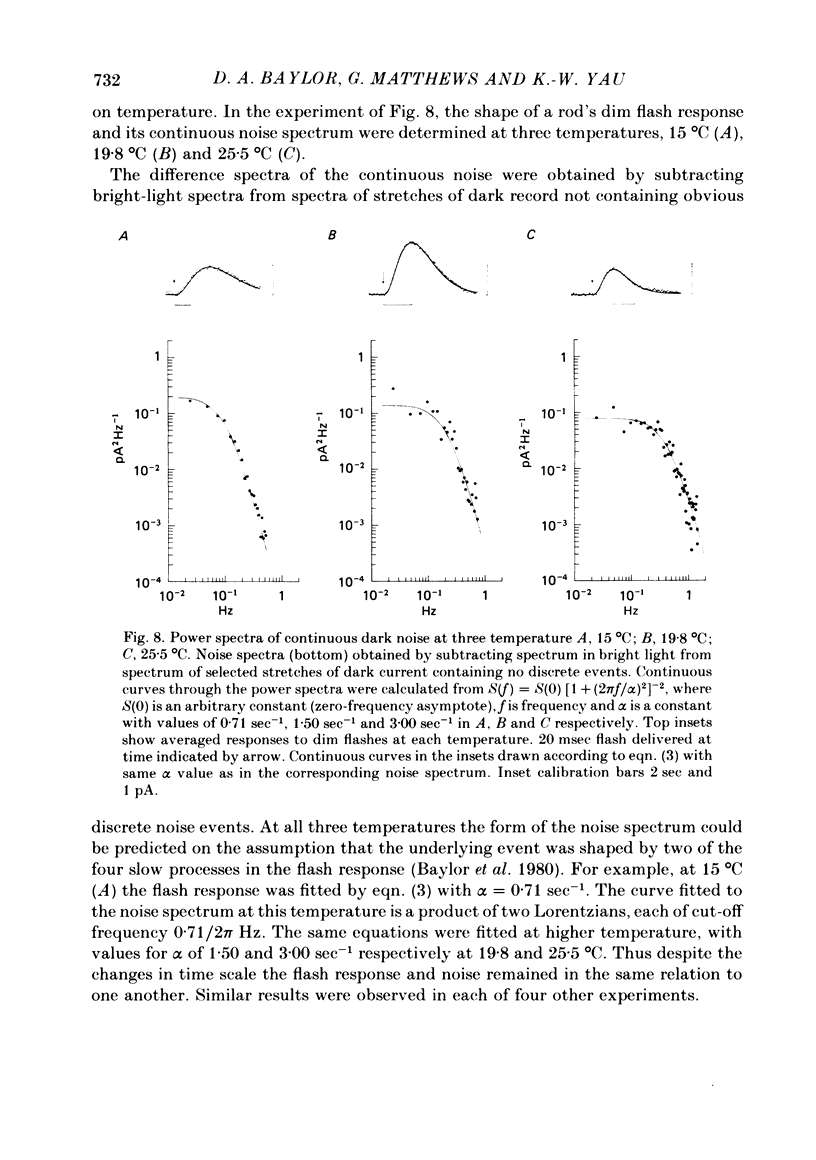
Abstract
Thermal effects on the visual transduction mechanism of toad rods were examined by recording the membrane current of a single outer segment while changing the temperature within the range 15-30 degrees C. Warming increased the amplitude rmax of the saturating flash response. This effect had a Q10 of about 1.8 and may result from an increase in the light-sensitive conductance. The flash sensitivity decreased with increasing temperature, while the half-saturating flash intensity increased. There was no evidence of a temperature effect on the probability that an incident 500 nm photon triggered an electrical response. Together with the results in (2) and (3) this indicates that at higher temperature a successfully absorbed photon blocked a smaller fraction of the light-sensitive conductance. Upon warming, the time scale of the flash response shortened but the characteristic wave form was preserved. The speed of the dim flash response, measured by the reciprocal of its time-to-peak, had a Q10 of 2.7 and an apparent activation energy of 16.8 kcal mole-1. The power spectrum of the continuous component of the dark noise could be predicted at different temperatures by assuming that the underlying event was shaped by two of the four delays required to fit the light response. This behaviour is consistent with the notion that the continuous noise arises within the cascade of processes controlling the internal transmitter concentration of the outer segment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENTON E. J., PIRENNE M. H. The visual sensitivity of the toad Xenopus laevis. J Physiol. 1954 Jul 28;125(1):181–207. doi: 10.1113/jphysiol.1954.sp005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ST GEORGE R. C. C. The interplay o light and heat in bleaching rhodopsin. J Gen Physiol. 1952 Jan;35(3):495–517. doi: 10.1085/jgp.35.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebro R. A thermal component of excitation in the lateral eye of Limulus. J Physiol. 1966 Nov;187(2):417–425. doi: 10.1113/jphysiol.1966.sp008099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]


