Abstract
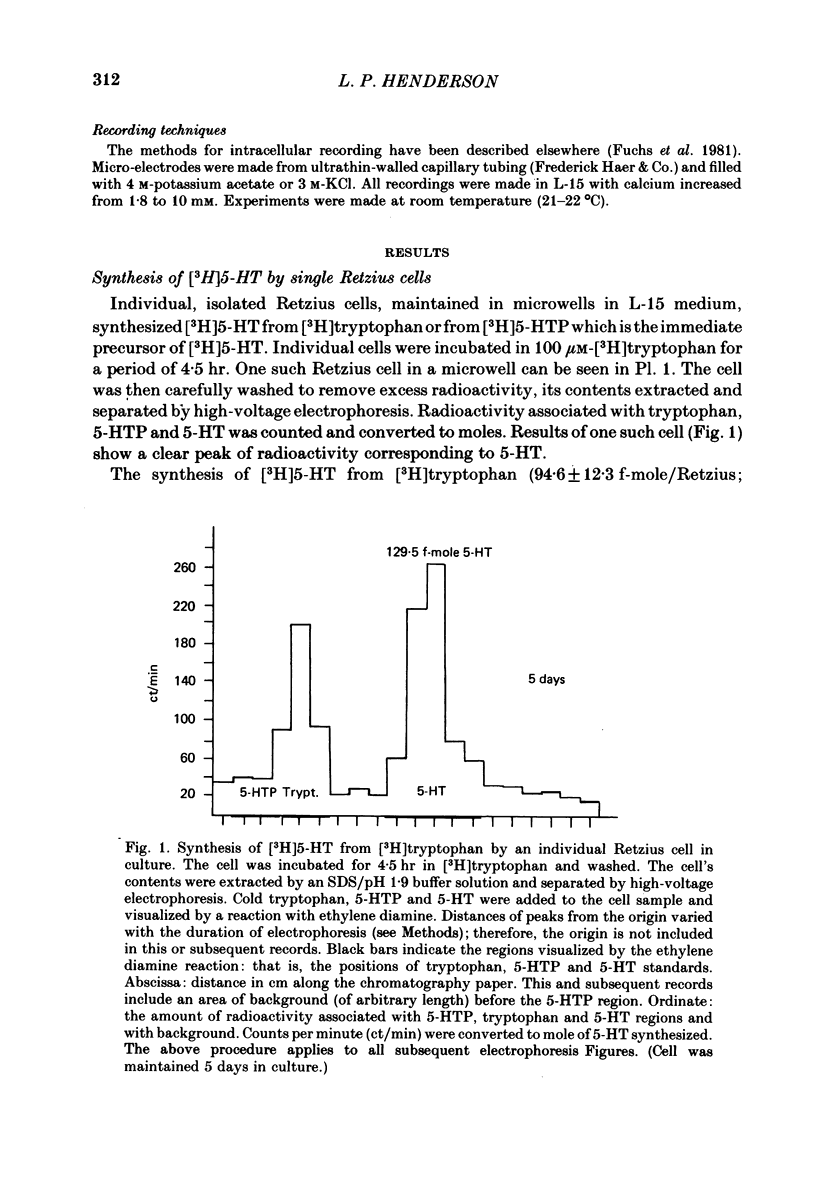
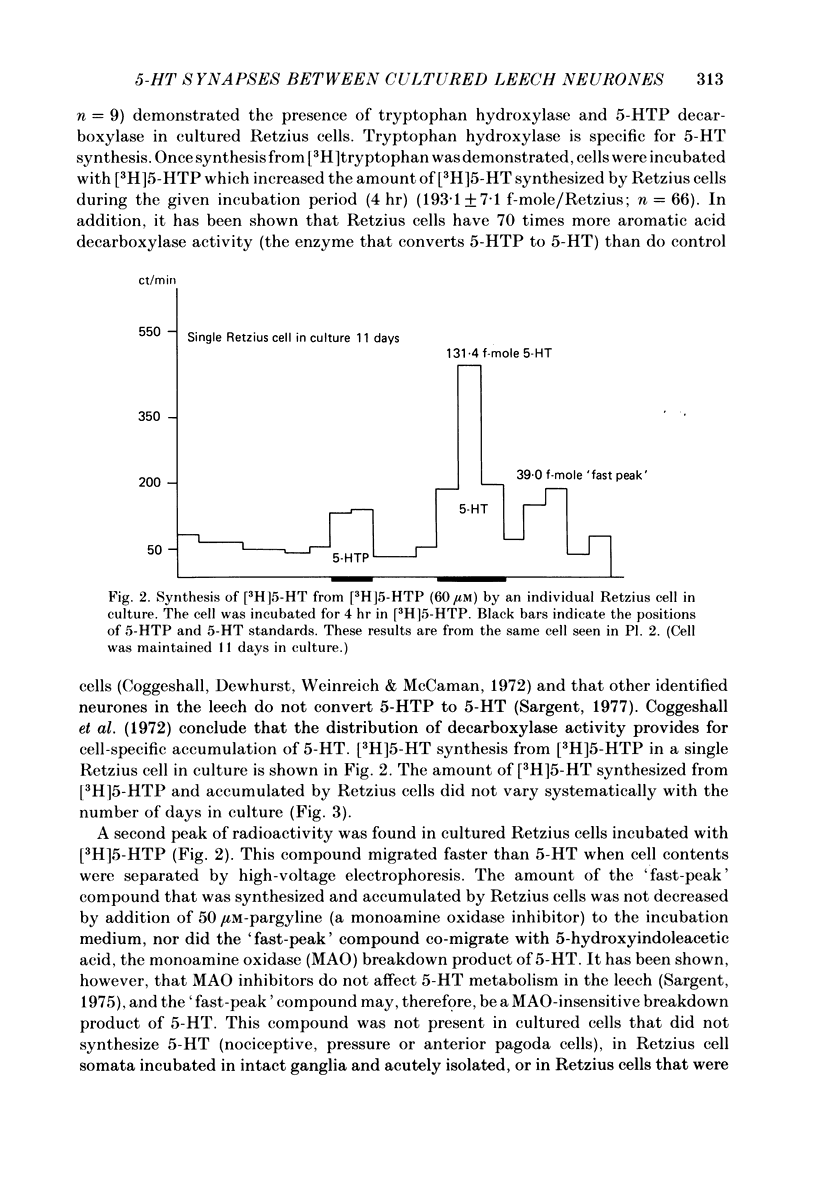
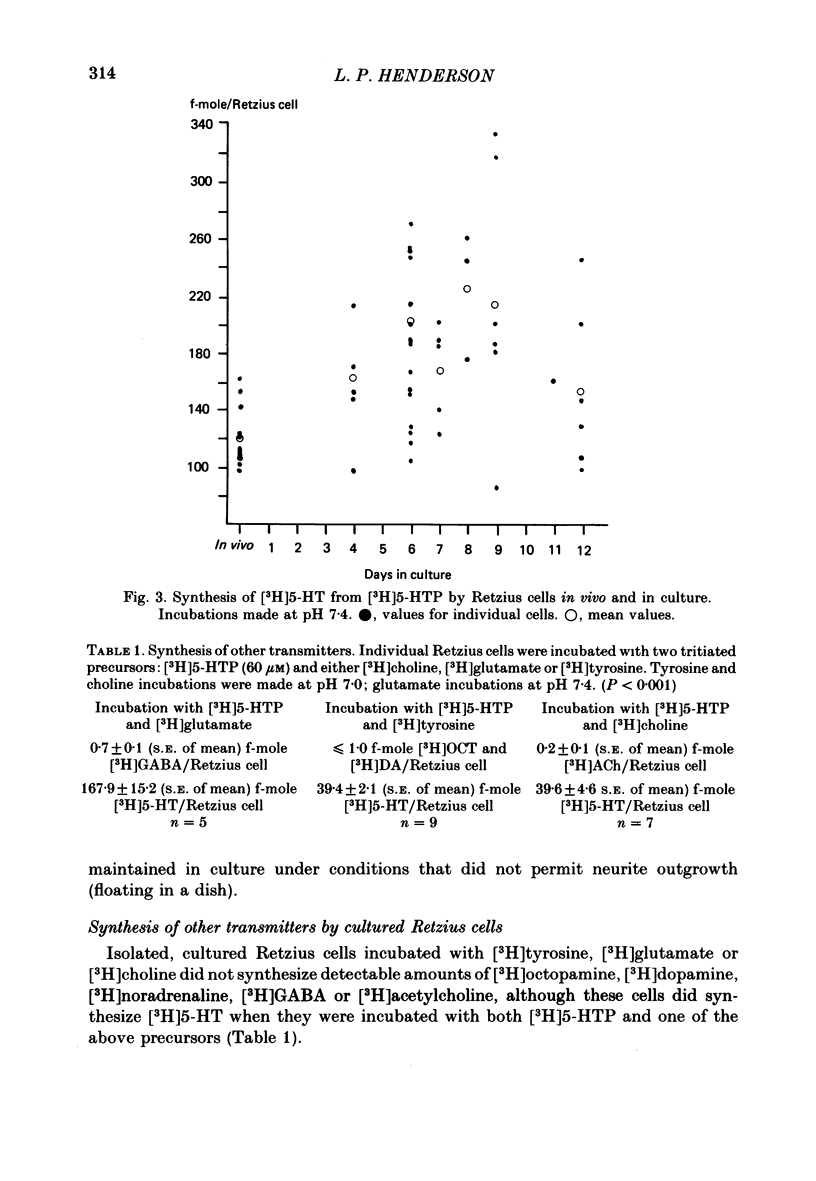
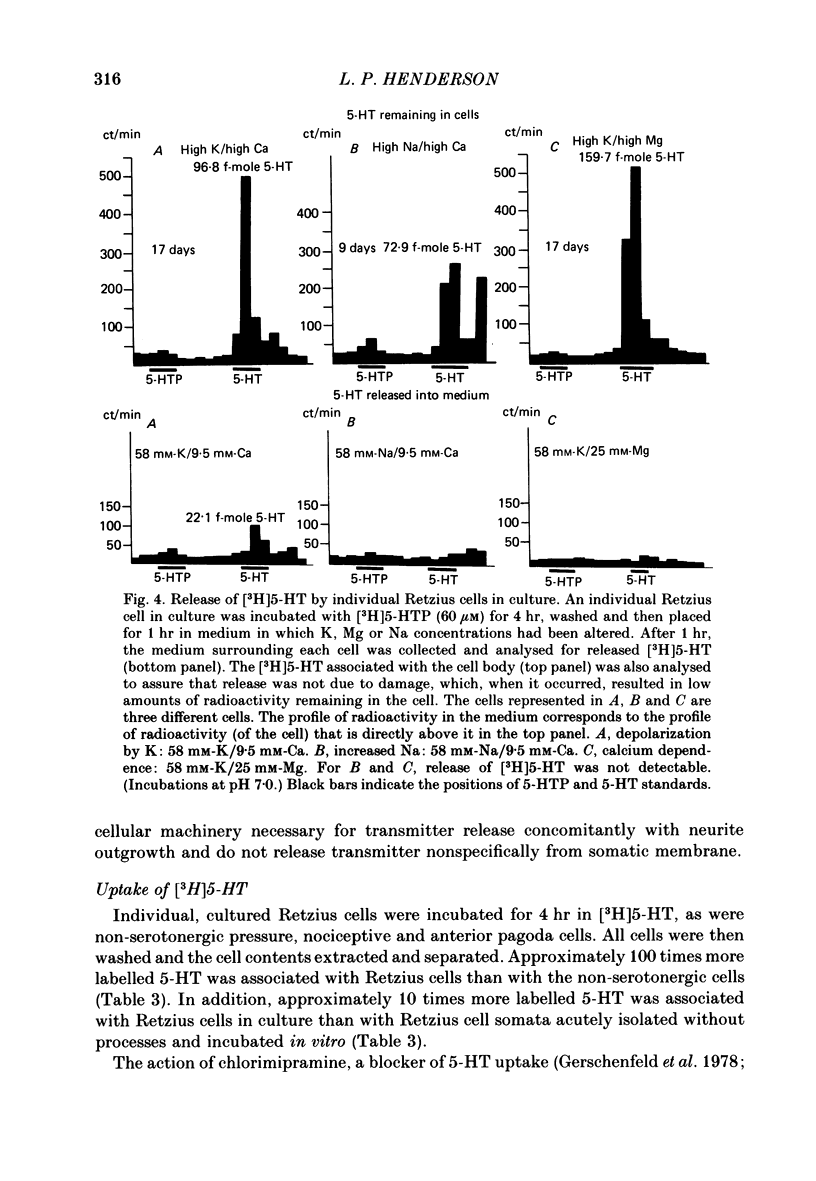
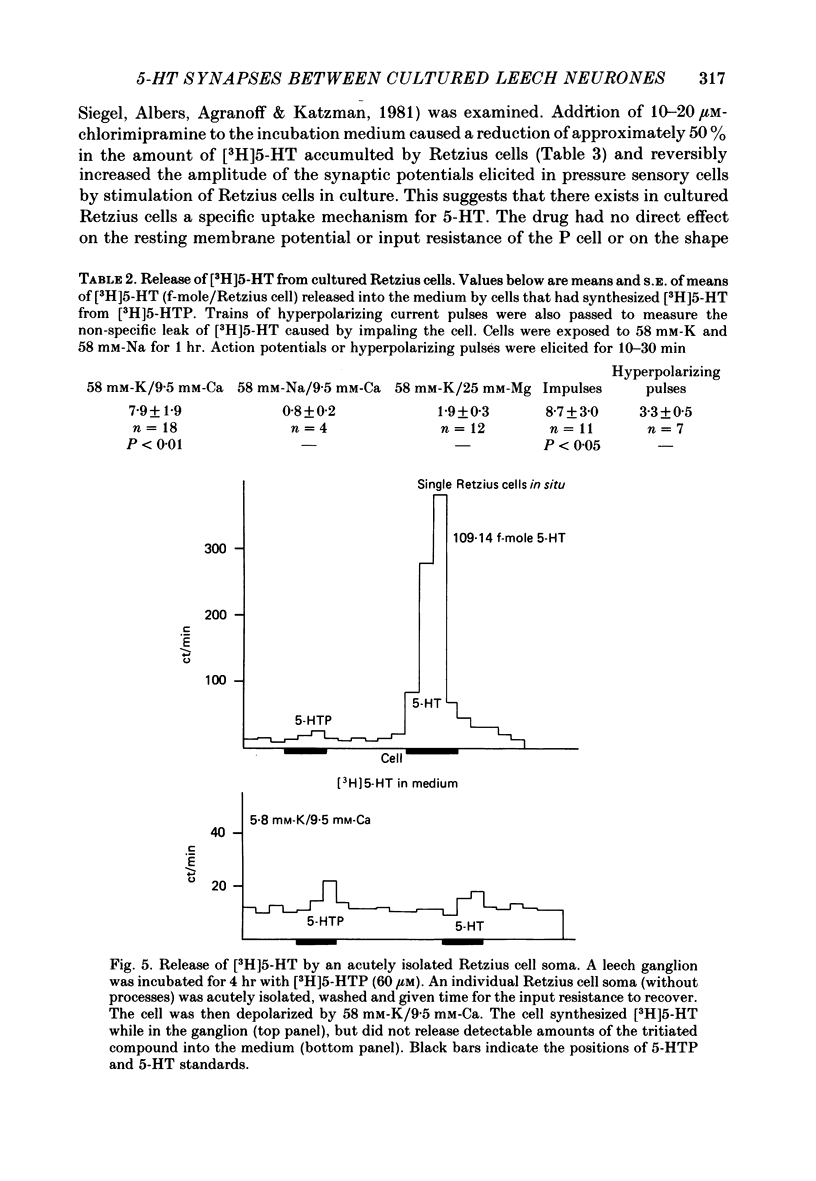
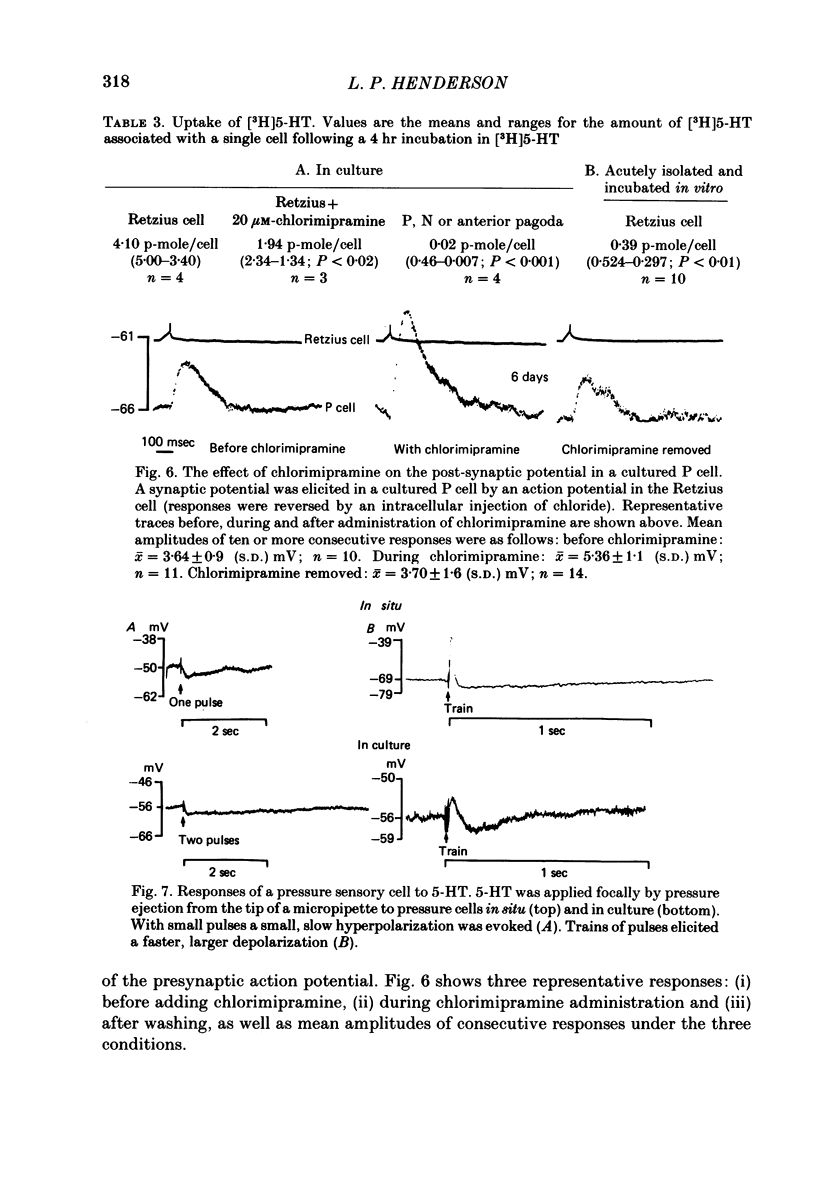
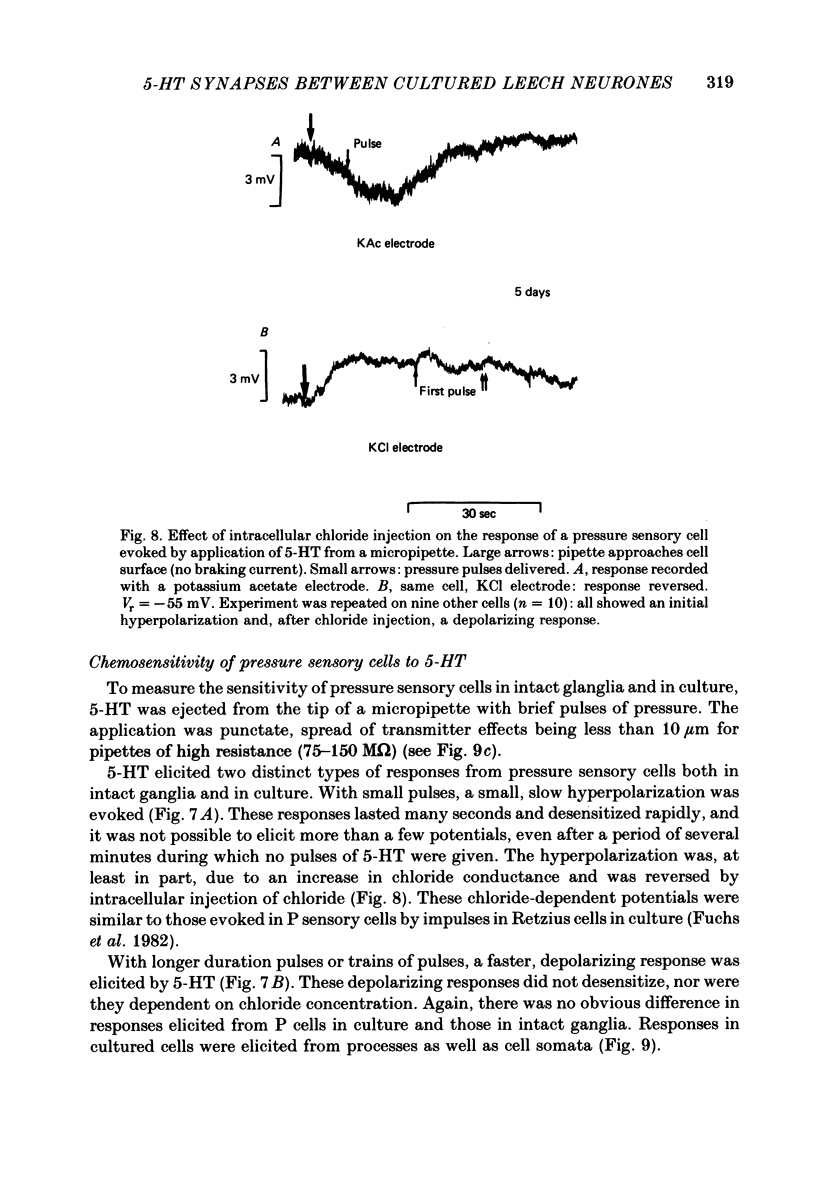
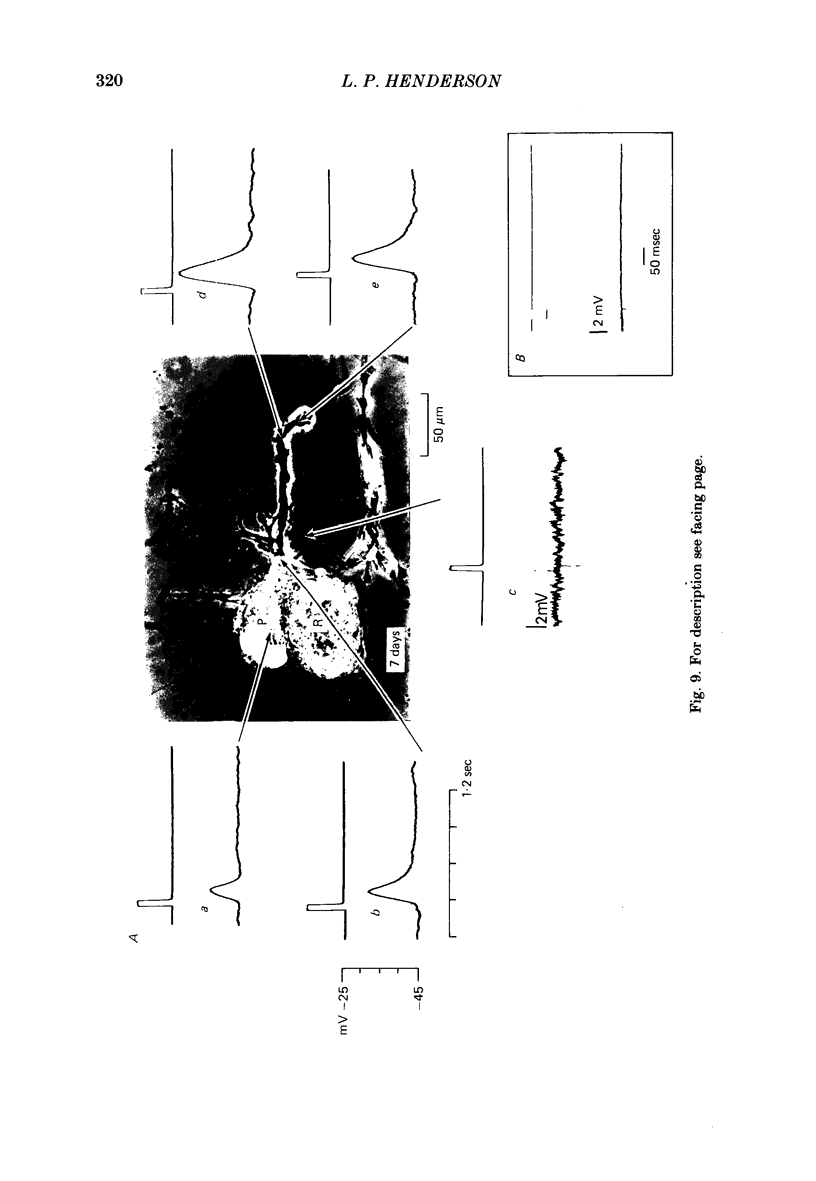
The synthesis, storage, release and synaptic actions of 5-hydroxytryptamine (5-HT or serotonin) were studied in order to characterize the synaptic connexion that develops between pairs of identified neurones dissected from the central nervous system of the leech and maintained in culture. Experiments were made with Retzius cells (which are known to contain 5-HT in vivo) and pressure sensory neurones on which they form chemical synapses in culture. Individual, isolated Retzius cells in culture synthesized [3H]5-HT from either [3H]tryptophan or [3H]5-hydroxytryptophan [( 3H]5-HTP). These cells did not synthesize other putative neurotransmitters, such as acetylcholine, dopamine, octopamine, noradrenaline or gamma-aminobutyric acid from their respective precursors. The monoaminergic character of Retzius cells was also demonstrated by staining with Neutral Red and by histofluorescence. Individual, isolated Retzius cells that had synthesized and accumulated [3H]5-HT released this compound when depolarized. Transmitter release was calcium-dependent and was blocked by magnesium. When incubated with [3H]5-HT and washed, Retzius cells in culture accumulated approximately 100 times more labelled 5-HT than did non-serotonergic cells, and 10 times more than Retzius cell somata acutely isolated from the animal and incubated in vitro. Chlorimipramine, a blocker of 5-HT uptake, decreased the amount of [3H]5-HT accumulated by Retzius cells and also caused a reversible increase in the amplitude of the synaptic response in the pressure sensory cell elicited by stimulation of the Retzius cell. Pressure sensory neurones in culture and in vivo responded to 5-HT focally applied by pressure ejection from a micropipette. Small pulses elicited a small, slow hyperpolarization. This response was due, at least in part, to an increase in chloride conductance and desensitized rapidly. With larger pulses, a larger, faster non-desensitizing depolarization was elicited. Together, these results provide evidence that 5-HT released from Retzius cells could be responsible for the chemical synaptic potentials seen in pressure sensory neurones in culture.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coggeshall R. E., Dewhurst S. A., Weinreich D., McCaman R. E. Aromatic acid decarboxylase and choline acetylase activities in a single identified 5-HT containing cell of the leech. J Neurobiol. 1972;3(3):259–265. doi: 10.1002/neu.480030308. [DOI] [PubMed] [Google Scholar]
- Fuchs P. A., Henderson L. P., Nicholls J. G. Chemical transmission between individual Retzius and sensory neurones of the leech in culture. J Physiol. 1982 Feb;323:195–210. doi: 10.1113/jphysiol.1982.sp014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Nicholls J. G., Ready D. F. Membrane properties and selective connexions of identified leech neurones in culture. J Physiol. 1981 Jul;316:203–223. doi: 10.1113/jphysiol.1981.sp013783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Hamon M., Paupardin-Tritsch D. Release of endogenous serotonin from two identified serotonin-containing neurones and the physiological role of serotonin re-uptake. J Physiol. 1978 Jan;274:265–278. doi: 10.1113/jphysiol.1978.sp012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie P. B., Neuhoff V., Osborne N. N. Monoamine oxidase activity in Helix pomatia. Experientia. 1975 Jul 15;31(7):775–776. doi: 10.1007/BF01938457. [DOI] [PubMed] [Google Scholar]
- Hildebrand J. G., Barker D. L., Herbert E., Kravitz E. A. Screening for neurotransmitters: a rapid radiochemical procedure. J Neurobiol. 1971;2(3):231–246. doi: 10.1002/neu.480020305. [DOI] [PubMed] [Google Scholar]
- Lent C. M. Retzius cells: neuroeffectors controlling mucus release by the leech. Science. 1973 Feb 16;179(4074):693–696. doi: 10.1126/science.179.4074.693. [DOI] [PubMed] [Google Scholar]
- Mason A., Sunderland A. J., Leake L. D. Effects of leech Retzius cells on body wall muscles. Comp Biochem Physiol C. 1979;63C(2):359–361. doi: 10.1016/0306-4492(79)90086-8. [DOI] [PubMed] [Google Scholar]
- McCaman M. W., Weinreich D., McCaman R. E. The determination of picomole levels of 5-hydroxytryptamine and dopamine in Aplysia, Tritonia and leech nervous tissues. Brain Res. 1973 Apr 13;53(1):129–137. doi: 10.1016/0006-8993(73)90772-5. [DOI] [PubMed] [Google Scholar]
- O'Lague P. H., MacLeish P. R., Nurse C. A., Claude P., Furshpan E. J., Potter D. D. Physiological and morphological studies on developing sympathetic neurons in dissociated cell culture. Cold Spring Harb Symp Quant Biol. 1976;40:399–407. doi: 10.1101/sqb.1976.040.01.038. [DOI] [PubMed] [Google Scholar]
- Osborne N. W., Briel G., Neuhoff V. The amine and amino acid composition in the Retzius cells of the leech Hirudo medicinalis. Experientia. 1972 Sep 15;28(9):1015–1018. doi: 10.1007/BF01918644. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Iversen L. L., Hall Z. W., Kravitz E. A. Release of gamma-aminobutyric acid from inhibitory nerves of lobster. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1110–1115. doi: 10.1073/pnas.56.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. I. Effects of conditioned medium. Dev Biol. 1977 Apr;56(2):263–280. doi: 10.1016/0012-1606(77)90269-x. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. II. Developmental aspects. Dev Biol. 1977 Oct 15;60(2):473–481. doi: 10.1016/0012-1606(77)90144-0. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Reichardt L. F., Chun L. L. Biochemical studies on the development of primary sympathetic neurons in cell culture. Cold Spring Harb Symp Quant Biol. 1976;40:389–397. doi: 10.1101/sqb.1976.040.01.037. [DOI] [PubMed] [Google Scholar]
- Pentreath V. W., Cottrell G. A. Uptake of serotonin, 5-hydroxytryptophan and tryptophan by giant serotonin-containing neurones and other neurones in the central nervous system of the snail (Helix pomatia). Z Zellforsch Mikrosk Anat. 1973;143(1):21–35. doi: 10.1007/BF00307448. [DOI] [PubMed] [Google Scholar]
- Ready D. F., Nicholls J. Identified neurones isolated from leech CNS make selective connections in culture. Nature. 1979 Sep 6;281(5726):67–69. doi: 10.1038/281067a0. [DOI] [PubMed] [Google Scholar]
- Rude S., Coggeshall E., Van Orden L. S., 3rd Chemical and ultrastructural identification of 5-hydroxytryptamine in an identified neuron. J Cell Biol. 1969 Jun;41(3):832–854. doi: 10.1083/jcb.41.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent P. B. Synthesis of acetylcholine by excitatory motoneurons in central nervous system of the leech. J Neurophysiol. 1977 Mar;40(2):453–460. doi: 10.1152/jn.1977.40.2.453. [DOI] [PubMed] [Google Scholar]
- Sargent P. B., Yau K. W., Nicholls J. G. Extrasynaptic receptors on cell bodies of neurons in central nervous system of the leech. J Neurophysiol. 1977 Mar;40(2):446–452. doi: 10.1152/jn.1977.40.2.446. [DOI] [PubMed] [Google Scholar]
- Stuart A. E., Hudspeth A. J., Hall Z. W. Vital staining of specific monoamine-containing cells in the leech nervous system. Cell Tissue Res. 1974;153(1):55–61. doi: 10.1007/BF00225445. [DOI] [PubMed] [Google Scholar]
- Torre J. C., Surgeon J. W. A methodological approach to rapid and sensitive monoamine histofluorescence using a modified glyoxylic acid technique: the SPG method. Histochemistry. 1976 Oct 22;49(2):81–93. doi: 10.1007/BF00495672. [DOI] [PubMed] [Google Scholar]
- Willard A. L. Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J Neurosci. 1981 Sep;1(9):936–944. doi: 10.1523/JNEUROSCI.01-09-00936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]