Abstract
1. The influence of aldosterone upon water and sodium transport properties of the distal colon was studied in long-term adrenalectomy (11-29 days).
2. Six groups of rats were used: I, normal (control); II, adrenalectomized; III, adrenalectomized, acutely substituted with aldosterone (200 μg/kg 4 h); IV, adrenalectomized rats receiving aldosterone simultaneously with the specific inhibitor spironolactone (40 mg/kg within 4 h); V, adrenalectomized, substituted chronically with aldosterone (2 × 75 μg/kg day); VI, adrenalectomized, substituted chronically with dexamethasone (120 μg/kg day).
3. Distal colon segments were perfused in vivo with isotonic Ringer solution. In addition, a hypotonic electrolyte solution (Na+ 111 mM) was used in groups I and II.
4. In adrenalectomy (group II), net water absorption (Jv) was significantly decreased from (normal) 54·4 μl/h cm2±10·5 (n = 9) to 41·2 μl/h cm2±7·3 (n = 4), and net Na+ absorption (JNa) was decreased from 13·6 μmol/h cm2+3·5 to 8·5 μmol/h cm2±0·9 (isotonic perfusate). Similarly, Jv was decreased from 54·0 μl/h cm2±8·3 (n = 4) to 37·3 μl/h cm2±4·2 (n = 7), and JNa from 8·6 μmol/h cm2±2·1 to 4·2 μmol/h cm2±2·1 (hypotonic perfusate).
5. Acute aldosterone substitution in adrenalectomy (III) had no effect upon Jv (37·1 μl/h cm2±10·3; n = 5) but increased JNa to 10·3 μmol/h cm2±0·3.
6. The luminal Na+ steady-state concentration was higher in group II (11·2 mmol l-1±3·6; n = 6) than in group I (3·3 mmol l-1±1·4; n = 29). Acute aldosterone substitution restored this value to normal (3·0 mmol l-1±1·2; n = 4). The aldosterone effect was partly blocked by spironolactone: the Na+ steady-state concentration was 6·4 mmol/l±0·6 (n = 3) in group IV.
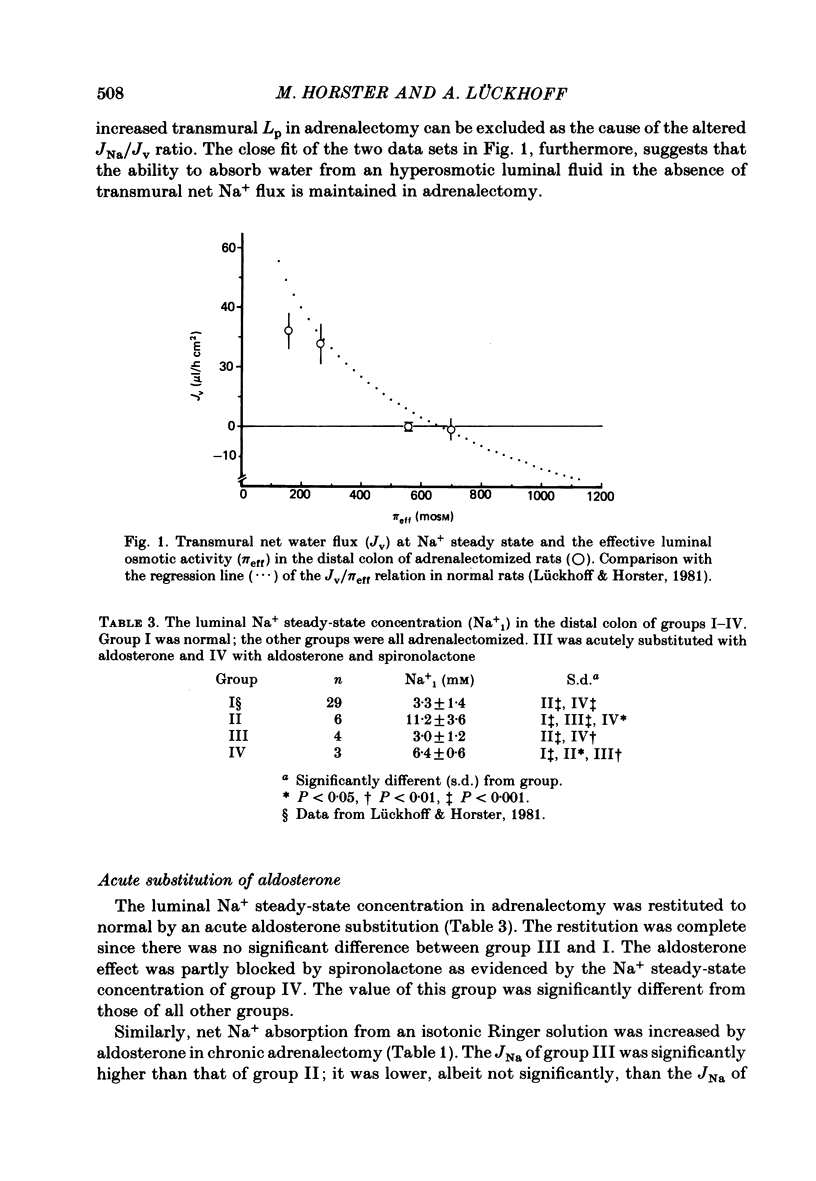
7. At the steady-state luminal Na+ concentration, the osmotically driven net water fluxes were not different in groups I and II, indicating that the hydraulic permeability coefficient is not altered in adrenalectomy.
8. In group V, Jv (54·9 μl/h cm2±10·9; n = 7) and JNa (11·9 μmol/h cm2±1·7; n = 6) were not significantly different from normal.
9. In group VI, Jv (37·3 μl/h cm2±6·0; n = 5) and JNa (8·0 μmol/h cm2±1·4) were not significantly different from group II.
10. The mineralocorticoid effects of aldosterone in long-term adrenalectomy appear to represent the principal determining factors of colonic Jv and JNa.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastl C. P., Binder H. J., Hayslett J. P. Role of glucocorticoids and aldosterone in maintenance of colonic cation transport. Am J Physiol. 1980 Mar;238(3):F181–F186. doi: 10.1152/ajprenal.1980.238.3.F181. [DOI] [PubMed] [Google Scholar]
- Chignell C. F., Titus E. Effect of adrenal steroids on a Na+- and K+-requiring adenosine triphosphatase from rat kidney. J Biol Chem. 1966 Nov 10;241(21):5083–5089. [PubMed] [Google Scholar]
- Dolman D., Edmonds C. J. The effect of aldosterone and the renin-angiotensin system on sodium, potassium and chloride transport by proximal and distal rat colon in vivo. J Physiol. 1975 Sep;250(3):597–611. doi: 10.1113/jphysiol.1975.sp011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J., Marriott J. C. The effect of aldosterone and adrenalectomy on the electrical potential difference of rat colon and on the transport of sodium, potassium, chloride and bicarbonate. J Endocrinol. 1967 Dec;39(4):517–531. doi: 10.1677/joe.0.0390517. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J., Marriott J. Sodium transport and short-circuit current in rat colon in vivo and the effect of aldosterone. J Physiol. 1970 Nov;210(4):1021–1039. doi: 10.1113/jphysiol.1970.sp009255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Hegel U. Segmental heterogeneity of epithelial transport in rat large intestine. Pflugers Arch. 1978 Dec 15;378(1):71–83. doi: 10.1007/BF00581960. [DOI] [PubMed] [Google Scholar]
- KAGAWA C. M. Blocking the renal electrolyte effects of mineralocorticoids with an orally active steroidal spirolactone. Endocrinology. 1960 Jul;67:125–132. doi: 10.1210/endo-67-1-125. [DOI] [PubMed] [Google Scholar]
- Landon E. J., Jazab N., Forte L. Aldosterone and sodium-potassium-dependent ATPase activity of rat kidney membranes. Am J Physiol. 1966 Oct;211(4):1050–1056. doi: 10.1152/ajplegacy.1966.211.4.1050. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Horster M. Hydraulic permeability coefficient and sodium steady-state luminal concentration of the in vivo perfused rat distal colon. Pflugers Arch. 1981 Oct;391(4):301–305. doi: 10.1007/BF00581511. [DOI] [PubMed] [Google Scholar]
- SINGER B., STACK-DUNNE M. P. The secretion of aldosterone and corticosterone by the rat adrenal. J Endocrinol. 1955 Mar;12(2):130–145. doi: 10.1677/joe.0.0120130. [DOI] [PubMed] [Google Scholar]


