Abstract
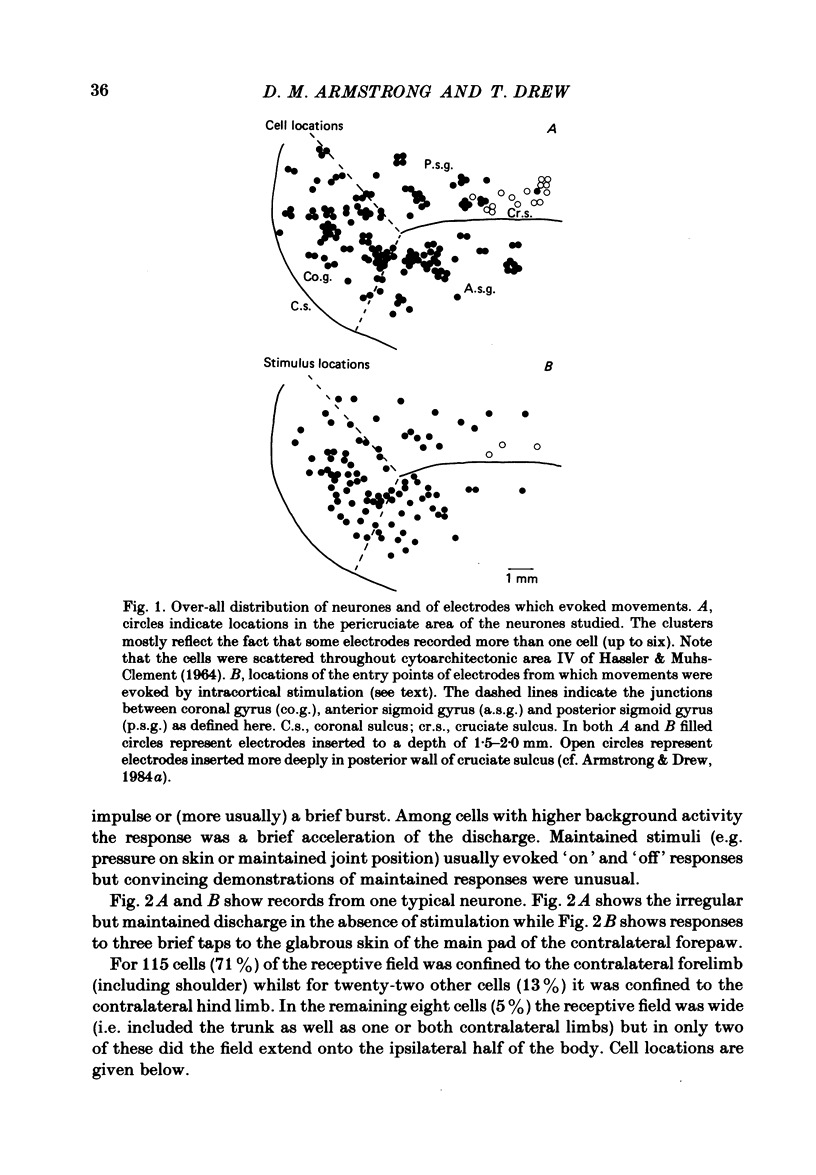
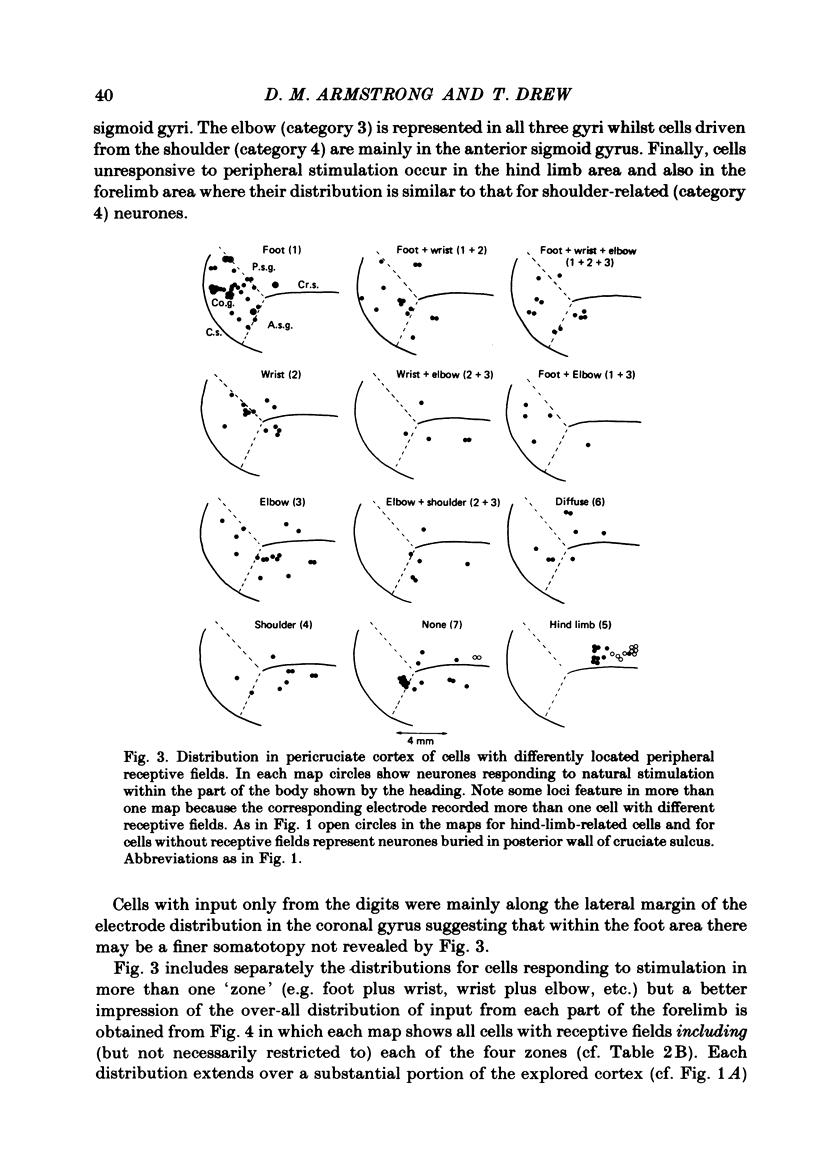
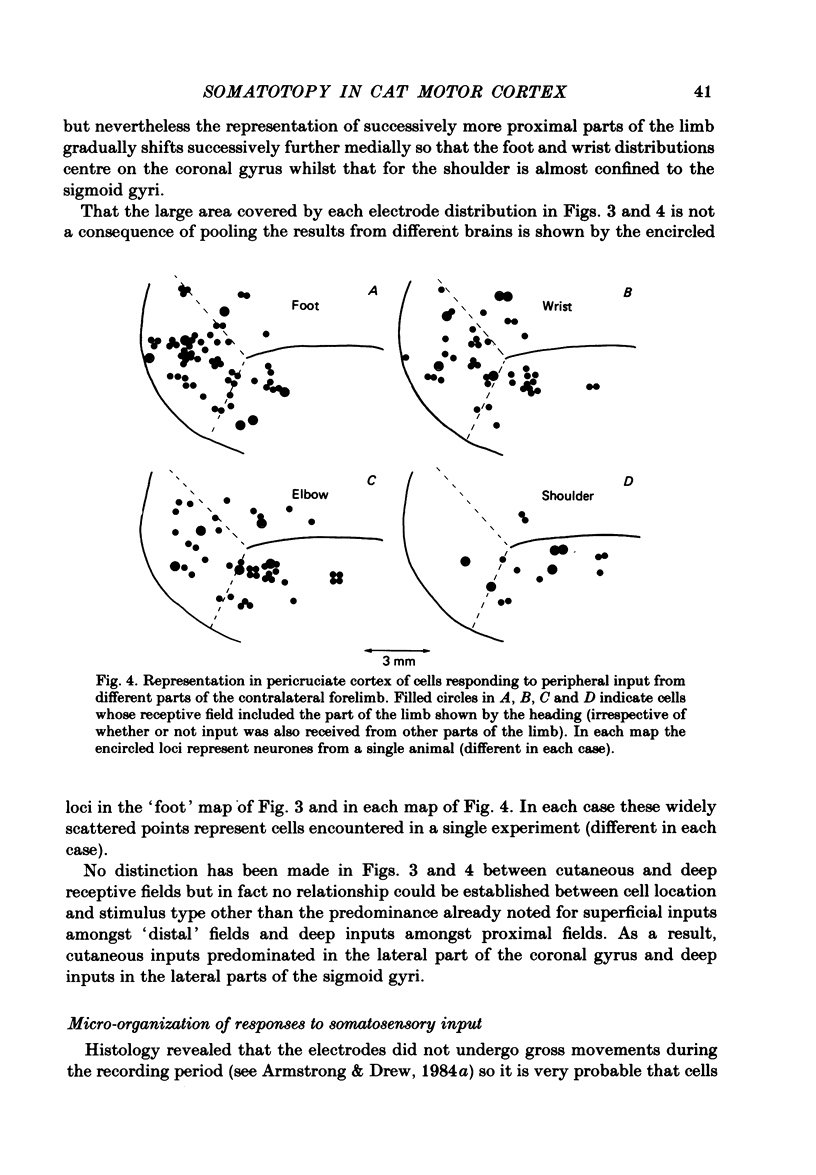
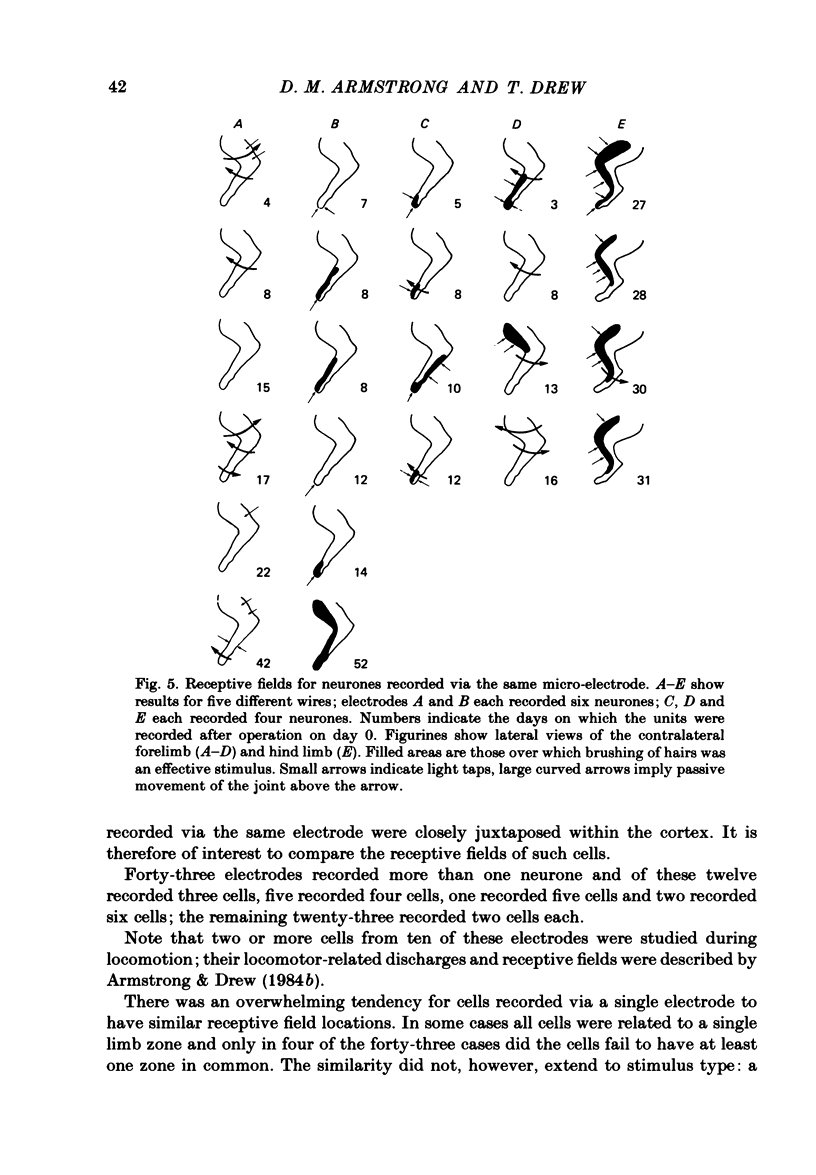
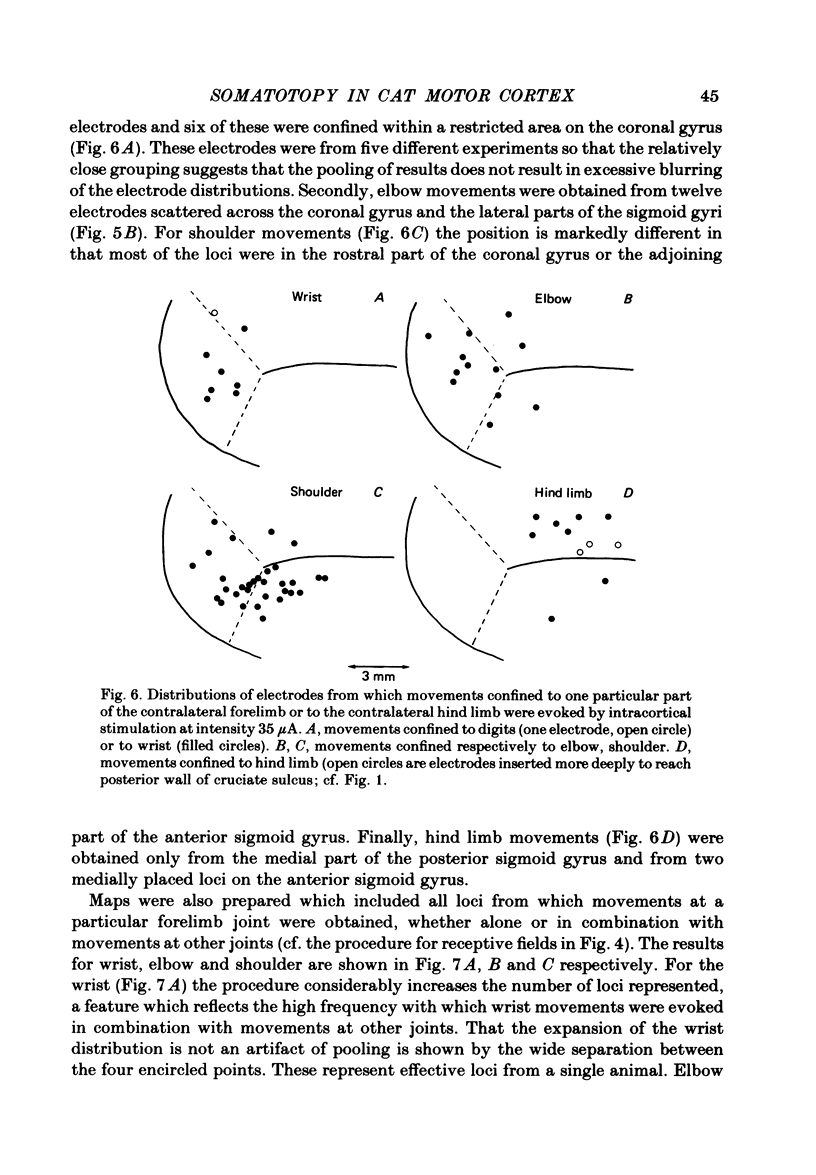
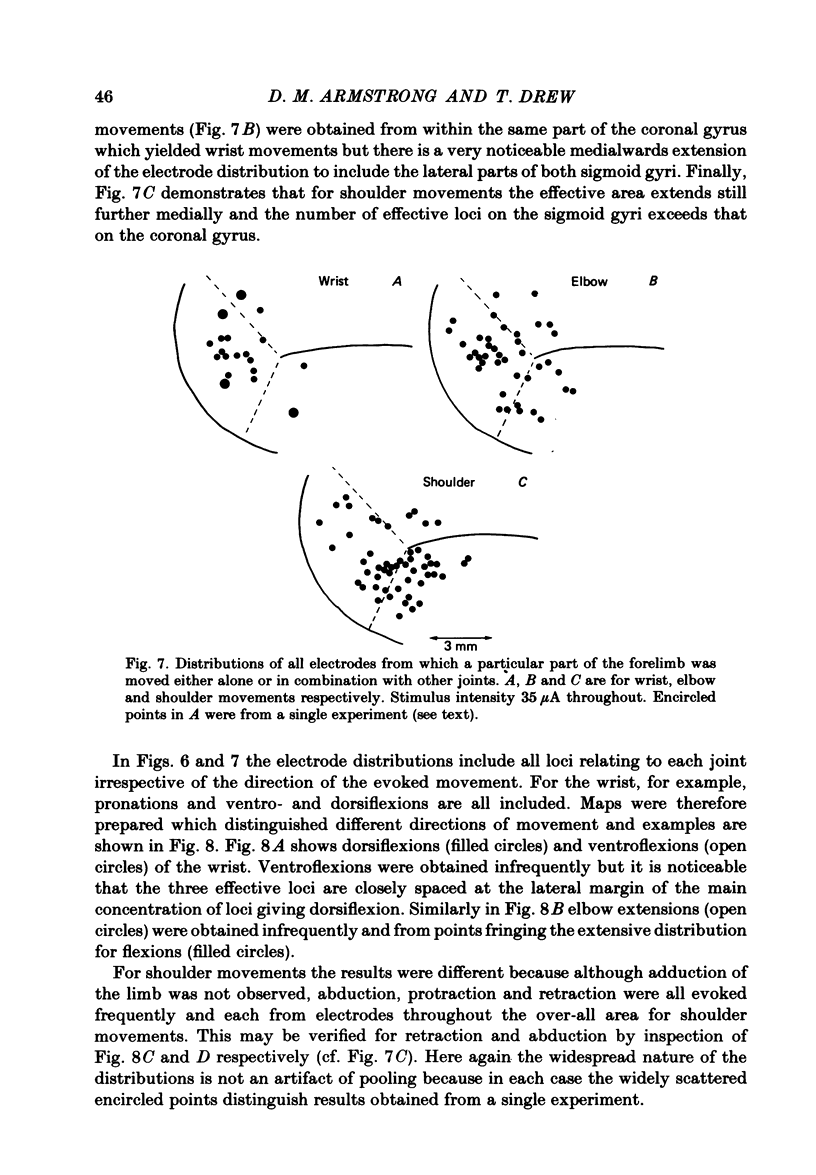
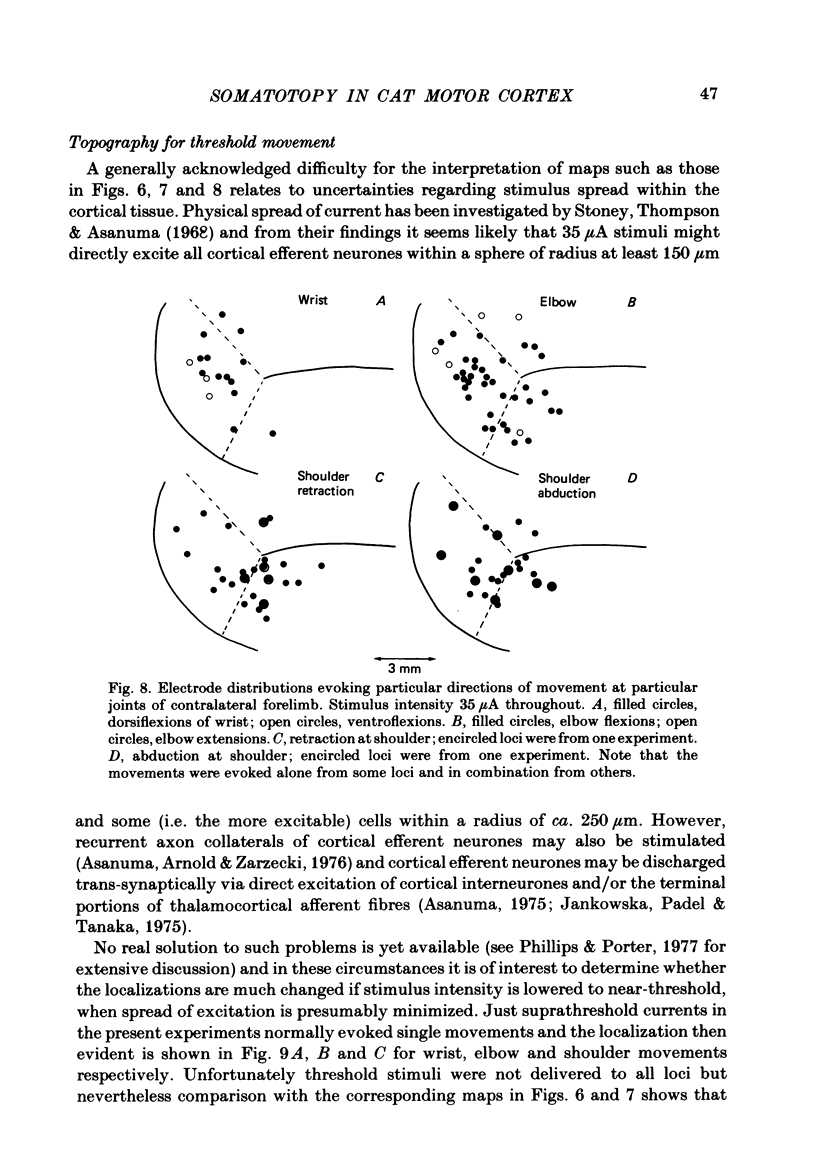
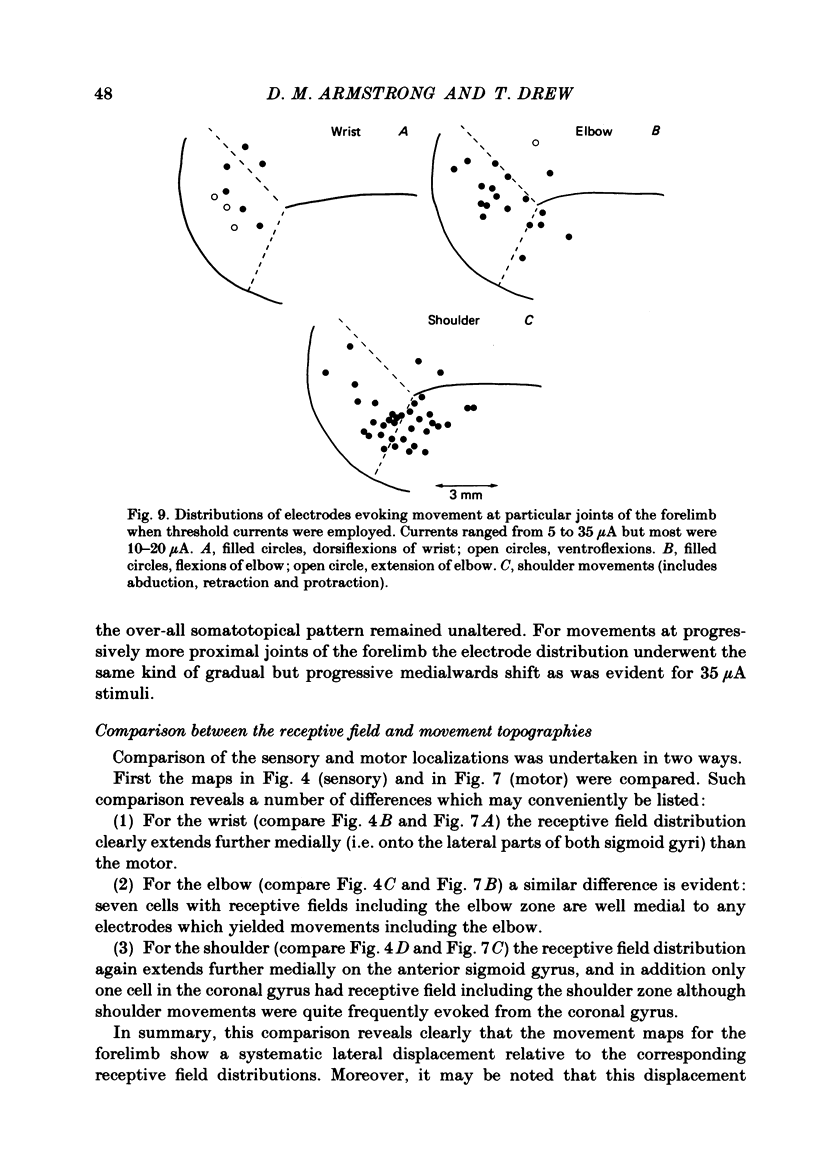
Microwires chronically implanted in the pericruciate cortex of free-to-move cats were used to record extracellularly from cortical neurones and to deliver intracortical stimulation. Natural stimulation of cutaneous and/or deep mechanoreceptors in limbs and trunk evoked discharges in 89% of 165 neurones, 57% of which were pyramidal tract neurones. Out of 112 cells with receptive fields on the contralateral forelimb, 41% had cutaneous fields, 29% had fields involving deep tissues and 30% were driven from both sources. Cutaneous receptive fields were much commoner than deep ones among cells with fields including the forefoot; this relationship was reversed for cells with more proximal fields. Many more cells had distal than proximal fields. The 'zones' of the forelimb (i.e. foot, wrist, elbow, shoulder) provided input to widespread and overlapping cell populations within the coronal gyrus and the lateral parts of the anterior and posterior sigmoid gyri. Despite the overlap a somatotopy existed with successively more distal limb zones represented successively further laterally in the pericruciate area. Intracortical stimulation (eleven cathodal pulses, duration 0.2 ms, frequency 330 Hz, intensity 35 microA or less) evoked flick movements of the contralateral limbs which were abolished by pyramidectomy. In the forelimb, shoulder movements were commonest and elbow, wrist and digits were represented with decreasing frequency. Both for 35 microA and for threshold stimulation the distributions of the effective electrodes revealed an overlapping somatotopy such that the wrist movements were almost restricted to the coronal gyrus and shoulder movements were most often evoked from the lateral part of the anterior sigmoid gyrus. The movement and receptive field somatotopies overlapped heavily but the former showed a distinct lateral shift relative to the latter. As a result shoulder movements were not uncommonly evoked from the coronal gyrus although the shoulder provided almost no input to cells in that area.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Drew T. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol. 1984 Jan;346:471–495. doi: 10.1113/jphysiol.1984.sp015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Locomotor-related neuronal discharges in cat motor cortex compared with peripheral receptive fields and evoked movements. J Physiol. 1984 Jan;346:497–517. doi: 10.1113/jphysiol.1984.sp015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H., Arnold A., Zarzecki P. Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Exp Brain Res. 1976 Dec 22;26(5):443–461. doi: 10.1007/BF00238820. [DOI] [PubMed] [Google Scholar]
- Asanuma H. Recent developments in the study of the columnar arrangement of neurons within the motor cortex. Physiol Rev. 1975 Apr;55(2):143–156. doi: 10.1152/physrev.1975.55.2.143. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Stoney S. D., Jr, Abzug C. Relationship between afferent input and motor outflow in cat motorsensory cortex. J Neurophysiol. 1968 Sep;31(5):670–681. doi: 10.1152/jn.1968.31.5.670. [DOI] [PubMed] [Google Scholar]
- Baker M. A., Tyner C. F., Towe A. L. Observations on single neurons recorded in the sigmoid gyri of awake, nonparalyzed cats. Exp Neurol. 1971 Sep;32(3):388–403. doi: 10.1016/0014-4886(71)90006-9. [DOI] [PubMed] [Google Scholar]
- Brooks V. B., Stoney S. D., Jr Motor mechanisms: the role of the pyramidal system in motor control. Annu Rev Physiol. 1971;33:337–392. doi: 10.1146/annurev.ph.33.030171.002005. [DOI] [PubMed] [Google Scholar]
- HASSLER R., MUHS-CLEMENT K. ARCHITEKTONISCHER AUFBAU DES SENSOMOTORISCHEN UND PARIETALEN CORTEX DE KATZE. J Hirnforsch. 1964;7:377–420. [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol. 1975 Aug;249(3):617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVINGSTON A., PHILLIPS C. G. Maps and thresholds for the sensorimotor cortex of the cat. Q J Exp Physiol Cogn Med Sci. 1957 Apr;42(2):190–205. doi: 10.1113/expphysiol.1957.sp001250. [DOI] [PubMed] [Google Scholar]
- Larsen K. D., Yumiya H. Organization of the convergence in the intermediate cerebellar nuclei of somatosensory receptive fields with motor cortical-evoked responses. Exp Brain Res. 1979 Aug 1;36(3):477–489. doi: 10.1007/BF00238517. [DOI] [PubMed] [Google Scholar]
- Lemon R. N. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. J Physiol. 1981 Feb;311:497–519. doi: 10.1113/jphysiol.1981.sp013601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon R. N. Variety of functional organization within the monkey motor cortex. J Physiol. 1981 Feb;311:521–540. doi: 10.1113/jphysiol.1981.sp013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A., Rispal-Padel L. Somatotopic localization in cat motor cortex. Brain Res. 1976 Apr 9;105(3):405–422. doi: 10.1016/0006-8993(76)90590-4. [DOI] [PubMed] [Google Scholar]
- Pappas C. L., Strick P. L. Physiological demonstration of multiple representation in the forelimb region of the cat motor cortex. J Comp Neurol. 1981 Aug 20;200(4):481–490. doi: 10.1002/cne.902000403. [DOI] [PubMed] [Google Scholar]
- Sakata H., Miyamoto J. Topographic relationship between the receptive fields of neurons in the motor cortex and the movements elicited by focal stimulation in freely moving cats. Jpn J Physiol. 1968 Aug 15;18(4):489–507. doi: 10.2170/jjphysiol.18.489. [DOI] [PubMed] [Google Scholar]
- Stoney S. D., Jr, Thompson W. D., Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol. 1968 Sep;31(5):659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Slow and fast groups of pyramidal tract cells and their respective membrane properties. J Neurophysiol. 1965 Sep;28(5):908–924. doi: 10.1152/jn.1965.28.5.908. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M. Input from muscle and cutaneous nerves of the hand and forearm to neurones of the precentral gyrus of baboons and monkeys. J Physiol. 1973 Jan;228(1):203–219. doi: 10.1113/jphysiol.1973.sp010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. C., Kwan H. C., MacKay W. A., Murphy J. T. Spatial organization of precentral cortex in awake primates. I. Somatosensory inputs. J Neurophysiol. 1978 Sep;41(5):1107–1119. doi: 10.1152/jn.1978.41.5.1107. [DOI] [PubMed] [Google Scholar]


