Abstract
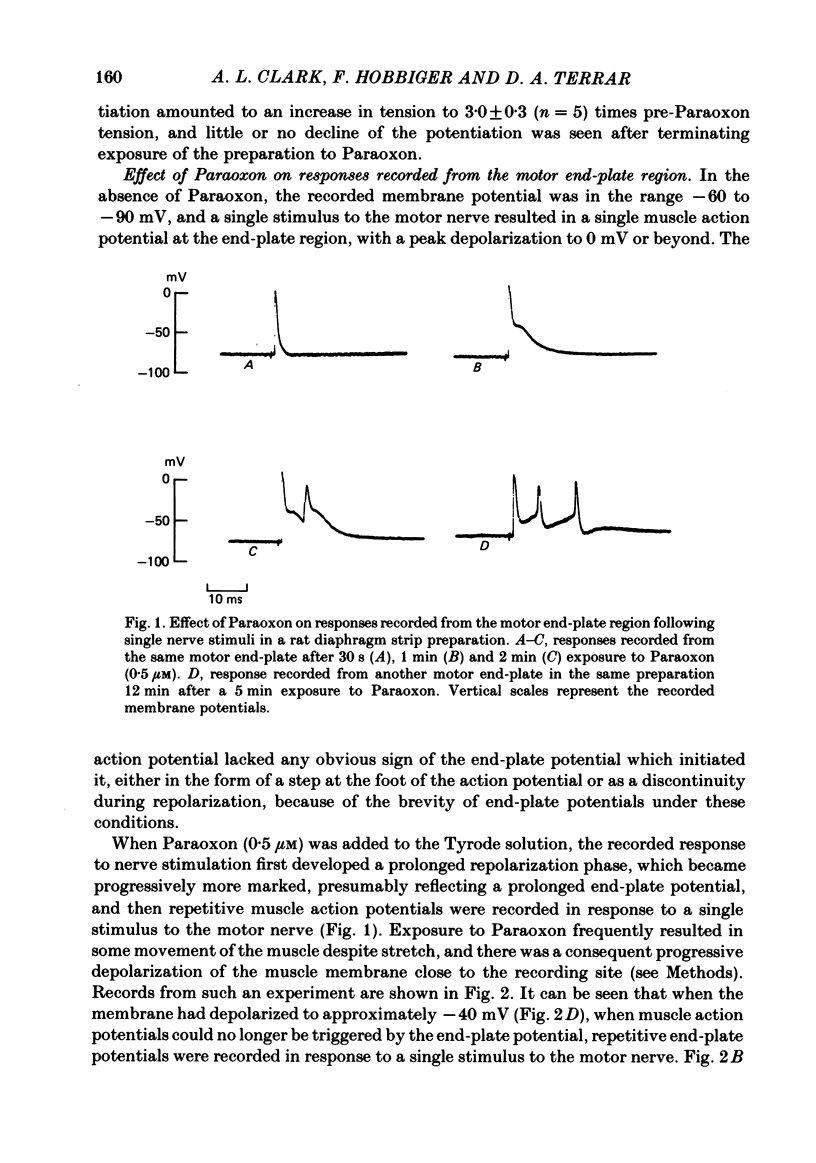
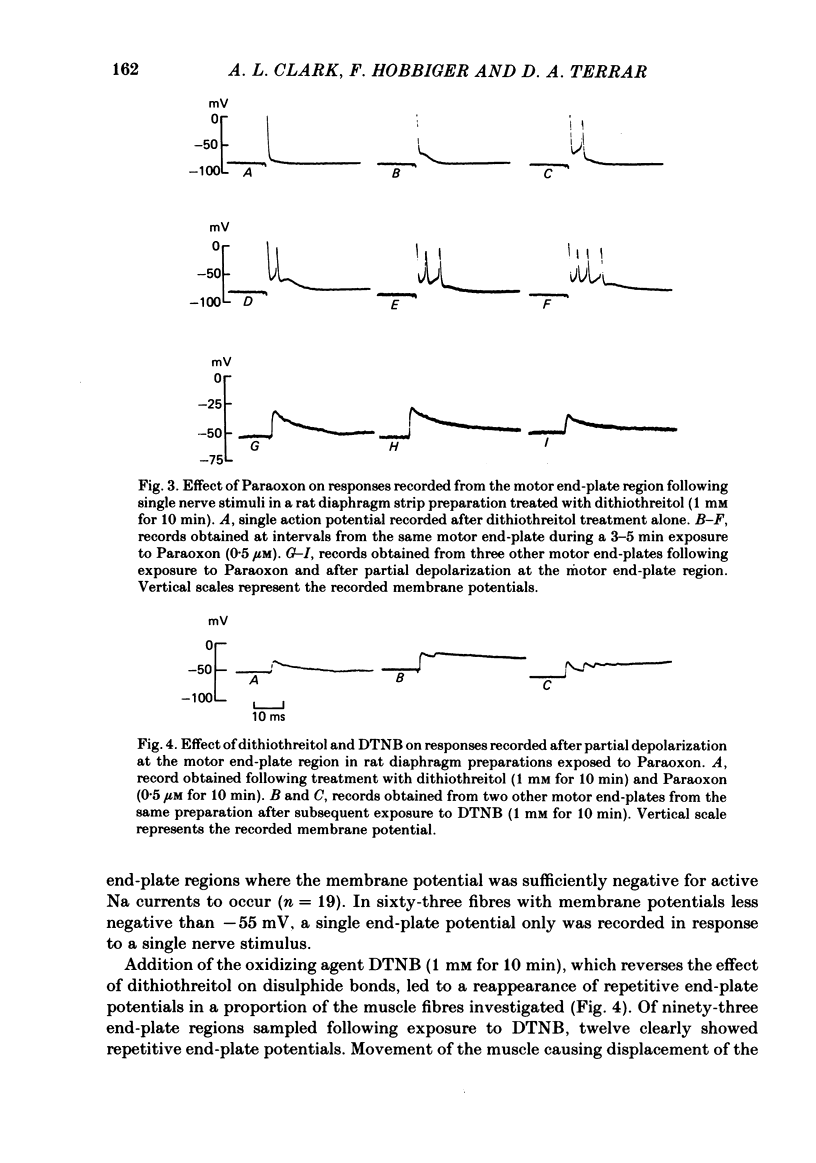
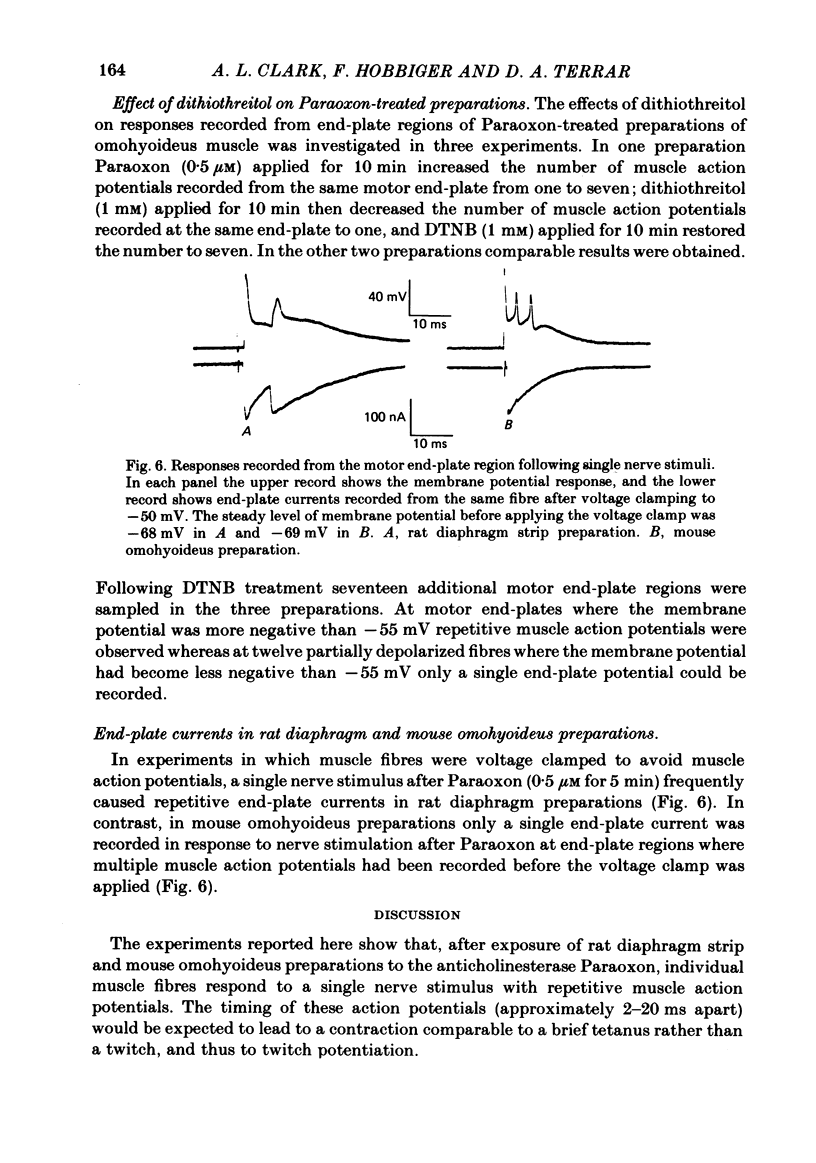
The action of the anticholinesterase Paraoxon on neuromuscular transmission in rat diaphragm and mouse omohyoideus preparations was investigated. In both preparations, when Paraoxon potentiated the twitch in response to a single nerve stimulus, repetitive muscle action potentials were recorded with an intracellular electrode placed at the motor end-plate region. At end-plates of Paraoxon-treated rat diaphragm preparations where the membrane potential was not sufficiently negative to support muscle action potentials, repetitive end-plate potentials were recorded in response to a single nerve stimulus. No repetitive end-plate potentials could be recorded under such conditions in preparations which had been exposed to dithiothreitol before being treated with Paraoxon, although twitch potentiation and repetitive muscle action potentials were still observed in these preparations. In Paraoxon-treated mouse omohyoideus preparations only single end-plate potentials were recorded from end-plates where the membrane potential was not sufficiently negative to support muscle action potentials. This applied whether or not the preparation had been treated with dithiothreitol before being exposed to Paraoxon. In voltage-clamped rat diaphragm preparations which had been treated with Paraoxon, repetitive end-plate currents were frequently recorded in response to a single nerve stimulus. Under the same conditions mouse omohyoideus preparations responded with a single end-plate current. It is concluded that Paraoxon-induced twitch potentiation in rat diaphragm and mouse omohyoideus preparations is caused by repetitive muscle action potentials being triggered by a single nerve stimulus. Under the conditions stated, the repetitive muscle action potentials in rat diaphragm preparations arose from a prolonged end-plate potential or repetitive end-plate potentials or a combination of both. In mouse omohyoideus preparations the repetitive muscle potentials were the consequence of a single prolonged end-plate potential.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Marshall M. W. Sodium currents in mammalian muscle. J Physiol. 1977 Jun;268(1):223–250. doi: 10.1113/jphysiol.1977.sp011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L. Action potentials of normal mammalian muscle. Effects of acetylcholine and eserine. J Physiol. 1937 Mar 5;89(2):220–237. doi: 10.1113/jphysiol.1937.sp003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. L., Hobbiger F., Terrar D. A. The effect of dithiothreitol on anticholinesterase induced antidromic firing and twitch potentiation [proceedings]. Br J Pharmacol. 1979 Nov;67(3):481P–482P. [PMC free article] [PubMed] [Google Scholar]
- Clark A. L., Hobbiger F., Terrar D. A. The relationship between stimulus-induced antidromic firing and twitch potentiation produced by paraoxon in rat phrenic nerve-diaphragm preparations. Br J Pharmacol. 1983 Sep;80(1):17–25. doi: 10.1111/j.1476-5381.1983.tb11044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. J Physiol. 1971 Feb;213(1):1–19. doi: 10.1113/jphysiol.1971.sp009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Müller K. D., Peper K., Sterz R. The M. omohyoideus of the mouse as a convenient mammalian muscle preparation. A study of junctional and extrajunctional acetylcholine receptors by noise analysis and cooperativity. Pflugers Arch. 1976 Dec 28;367(2):115–122. doi: 10.1007/BF00585146. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappone P. A. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol. 1980 Sep;306:377–410. doi: 10.1113/jphysiol.1980.sp013403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Terrar D. A. Effects of dithiothreitol on end-plate currents. J Physiol. 1978 Mar;276:403–417. doi: 10.1113/jphysiol.1978.sp012243. [DOI] [PMC free article] [PubMed] [Google Scholar]


