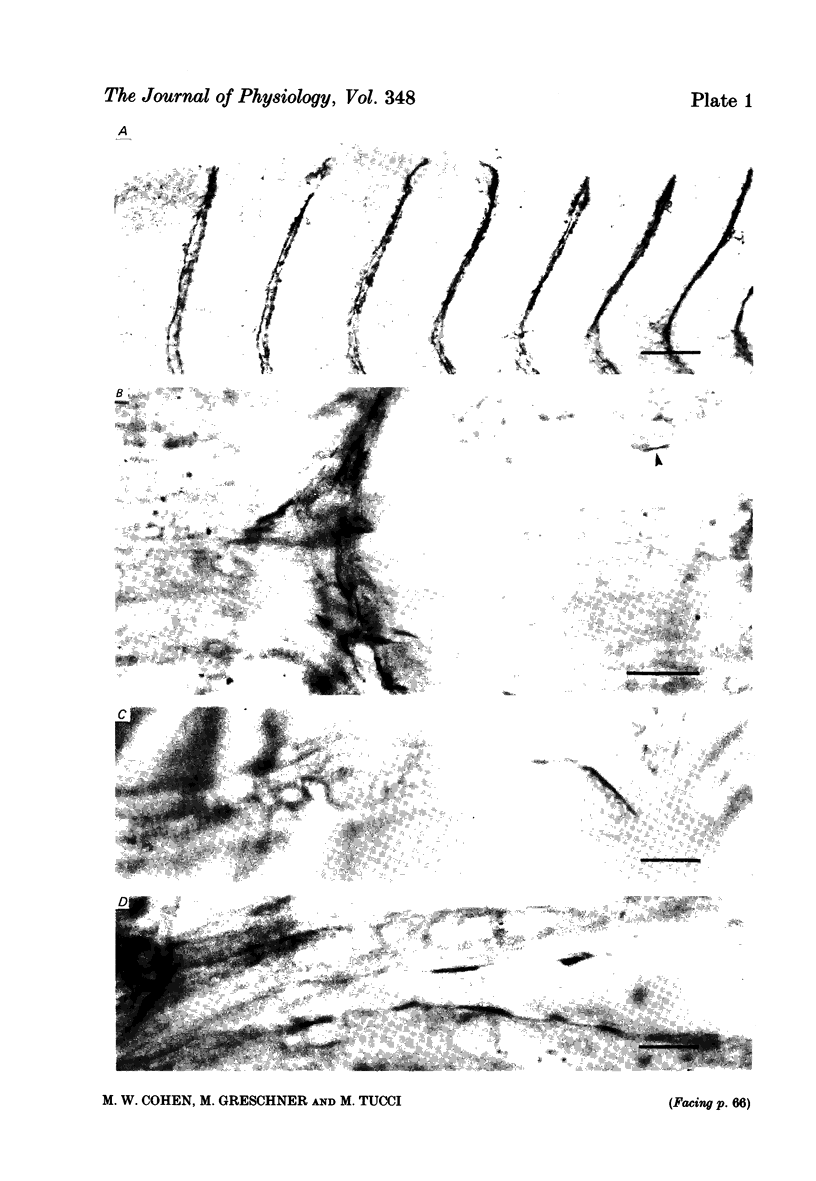
Abstract
Embryos of Xenopus laevis were selected prior to the onset of innervation and were raised for 2 days in the anaesthetic tricaine methanesulphonate (200 micrograms/ml). The gross development of these tricaine-reared animals appeared normal despite the absence of spontaneous motor activity and the lack of motor responses to prodding with a pin. Motor activity quickly appeared when the anaesthetic was withdrawn. Intracellular recording from the myotomes of intact, tricaine-maintained animals failed to reveal any spontaneous muscle action potentials. Synaptic potentials increased in frequency and amplitude upon withdrawing tricaine, but resting potentials remained unchanged. Cholinesterase activity, detected histochemically, was observed at the ends of the myotomes, the main site of innervation. The intensity of the histochemical reaction product at these sites appeared to be about as great in the myotomes of tricaine-reared animals as in control myotomes. Miniature end-plate currents (m.e.p.c.s), examined by focal external recording, declined with a time constant of 2.9 +/- 0.2 ms (mean +/- S.E. of mean) in the myotomes of tricaine-reared animals (stages 40-41). The time constants in the myotomes of control animals were 1.8 +/- 0.1 ms at stages 40-41 and 8.7 +/- 0.7 ms at stages 24-26 (shortly after the onset of innervation). The anticholinesterase neostigmine doubled m.e.p.c. time constants in the myotomes of tricaine-reared animals as well as in control myotomes at stages 40-41. It is concluded that motor activity is not required for the in vivo development of physiological levels of synaptic cholinesterase in Xenopus myotomal muscle.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. J., Cohen M. W., Zorychta E. Effects of innervation on the distribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):731–756. doi: 10.1113/jphysiol.1977.sp011879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. L., Turin L., Warner A. E. Muscle activity and the loss of electrical coupling between striated muscle cells in Xenopus embryos. J Neurosci. 1983 Jul;3(7):1414–1421. doi: 10.1523/JNEUROSCI.03-07-01414.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H., Bourgeois J. P., Changeux J. P. Evolution of cholinergic proteins in developing slow and fast skeletal muscles in chick embryo. J Physiol. 1980 May;302:197–218. doi: 10.1113/jphysiol.1980.sp013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S. E., Warner A. E. Low resistance junctions between mesoderm cells during development of trunk muscles. J Physiol. 1976 Feb;255(1):209–230. doi: 10.1113/jphysiol.1976.sp011276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S., Warner A. Onset of acetylcholine sensitivity and endplate activity in developing myotome muscles of Xenopus. Nature. 1976 Jul 15;262(5565):217–218. doi: 10.1038/262217a0. [DOI] [PubMed] [Google Scholar]
- Chow I., Cohen M. W. Developmental changes in the distribution of acetylcholine receptors in the myotomes of Xenopus laevis. J Physiol. 1983 Jun;339:553–571. doi: 10.1113/jphysiol.1983.sp014733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. W. Development of an amphibian neuromuscular junction in vivo and in culture. J Exp Biol. 1980 Dec;89:43–56. doi: 10.1242/jeb.89.1.43. [DOI] [PubMed] [Google Scholar]
- Cohen M. W. The development of neuromuscular connexions in the presence of D-tubocurarine. Brain Res. 1972 Jun 22;41(2):457–463. doi: 10.1016/0006-8993(72)90515-x. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge L., Liebhold M., Steinbach J. H. Alterations in cat skeletal neuromuscular junctions following prolonged inactivity. J Physiol. 1981;313:529–545. doi: 10.1113/jphysiol.1981.sp013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier D. T., Narahashi T. Tricaine (MS-222): effects on ionic conductances of squid axon membranes. Eur J Pharmacol. 1975 Sep-Oct;33(2):313–317. doi: 10.1016/0014-2999(75)90175-2. [DOI] [PubMed] [Google Scholar]
- Giacobini G., Filogamo G., Weber M., Boquet P., Changeux J. P. Effects of a snake alpha-neurotoxin on the development of innervated skeletal muscles in chick embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1708–1712. doi: 10.1073/pnas.70.6.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Perry R., Tuffery A. R., Vrbová G G G. Possible mechanisms determining synapse formation in developing skeletal muscles of the chick. Cell Tissue Res. 1974;155(1):13–25. doi: 10.1007/BF00220281. [DOI] [PubMed] [Google Scholar]
- Guth L., Zalewski A. A., Brown W. C. Quantitative changes in cholinesterase activity of denervated sole plates following implantation of nerve into muscle. Exp Neurol. 1966 Oct;16(2):136–147. doi: 10.1016/0014-4886(66)90093-8. [DOI] [PubMed] [Google Scholar]
- Harris A. J. Embryonic growth and innervation of rat skeletal muscles. II. Neural regulation of muscle cholinesterase. Philos Trans R Soc Lond B Biol Sci. 1981 Jul 16;293(1065):279–286. doi: 10.1098/rstb.1981.0077. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Van Essen D. C. Re-innervation of rat skeleton muscle in the presence of alpha-bungarotoxin. J Physiol. 1975 Sep;250(3):651–667. doi: 10.1113/jphysiol.1975.sp011075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J. THE LOCALIZATION OF CHOLINESTERASE ACTIVITY IN RAT CARDIAC MUSCLE BY ELECTRON MICROSCOPY. J Cell Biol. 1964 Nov;23:217–232. doi: 10.1083/jcb.23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg R. W., Lentz T. L., Cohen M. W. Development of the myotomal neuromuscular junction in Xenopus laevis: an electrophysiological and fine-structural study. Dev Biol. 1977 Oct 1;60(1):101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Kullberg R. W., Mikelberg F. S., Cohen M. W. Contribution of cholinesterase to developmental decreases in the time course of synaptic potentials at an amphibian neuromuscular junction. Dev Biol. 1980 Mar 15;75(2):255–267. doi: 10.1016/0012-1606(80)90161-x. [DOI] [PubMed] [Google Scholar]
- Lømo T., Slater C. R. Control of junctional acetylcholinesterase by neural and muscular influences in the rat. J Physiol. 1980 Jun;303:191–202. doi: 10.1113/jphysiol.1980.sp013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody-Corbett F., Cohen M. W. Localization of cholinesterase at sites of high acetylcholine receptor density on embryonic amphibian muscle cells cultured without nerve. J Neurosci. 1981 Jun;1(6):596–605. doi: 10.1523/JNEUROSCI.01-06-00596.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody-Corbett F., Weldon P. R., Cohen M. W. Cholinesterase localization at sites of nerve contact on embryonic amphibian muscle cells in culture. J Neurocytol. 1982 Jun;11(3):381–394. doi: 10.1007/BF01257984. [DOI] [PubMed] [Google Scholar]
- Obata K. Development of neuromuscular transmission in culture with a variety of neurons and in the presence of cholinergic substances and tetrodotoxin. Brain Res. 1977 Jan 1;119(1):141–153. doi: 10.1016/0006-8993(77)90096-8. [DOI] [PubMed] [Google Scholar]
- Ohr E. A. Tricaine methanesulfonate--1. pH and its effects on anesthetic potency. Comp Biochem Physiol C. 1976;54(1):13–17. doi: 10.1016/0306-4492(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Pittman R., Gray M., Maderdrut J. L. Embryonic behavior, hatching and neuromuscular development in the chick following a transient reduction of spontaneous motility and sensory input by neuromuscular blocking agents. J Comp Neurol. 1978 Jun 1;179(3):619–640. doi: 10.1002/cne.901790310. [DOI] [PubMed] [Google Scholar]
- Rieger F., Koenig J., Vigny M. Spontaneous contractile activity and the presence of the 16 S form of acetylcholinesterase in rat muscle cells in culture: reversible suppressive action of tetrodotoxin. Dev Biol. 1980 May;76(2):358–365. doi: 10.1016/0012-1606(80)90385-1. [DOI] [PubMed] [Google Scholar]
- Rubin L. L., Schuetze S. M., Weill C. L., Fischbach G. D. Regulation of acetylcholinesterase appearance at neuromuscular junctions in vitro. Nature. 1980 Jan 17;283(5744):264–267. doi: 10.1038/283264a0. [DOI] [PubMed] [Google Scholar]
- Steinbach J. H. Neuromuscular junctions and alpha-bungarotoxin-binding sites in denervated and contralateral cat skeletal muscles. J Physiol. 1981;313:513–528. doi: 10.1113/jphysiol.1981.sp013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H. Role of muscle activity in nerve-muscle interaction in vitro. Nature. 1974 Mar 1;248(5443):70–71. doi: 10.1038/248070a0. [DOI] [PubMed] [Google Scholar]
- Walicke P. A., Patterson P. H. On the role of Ca2+ in the transmitter choice made by cultured sympathetic neurons. J Neurosci. 1981 Apr;1(4):343–350. doi: 10.1523/JNEUROSCI.01-04-00343.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Junctional form of acetylcholinesterase restored at nerve-free endplates. Dev Biol. 1979 Feb;68(2):631–635. doi: 10.1016/0012-1606(79)90233-1. [DOI] [PubMed] [Google Scholar]
- Weldon P. R., Cohen M. W. Development of synaptic ultrastructure at neuromuscular contacts in an amphibian cell culture system. J Neurocytol. 1979 Apr;8(2):239–259. doi: 10.1007/BF01175564. [DOI] [PubMed] [Google Scholar]