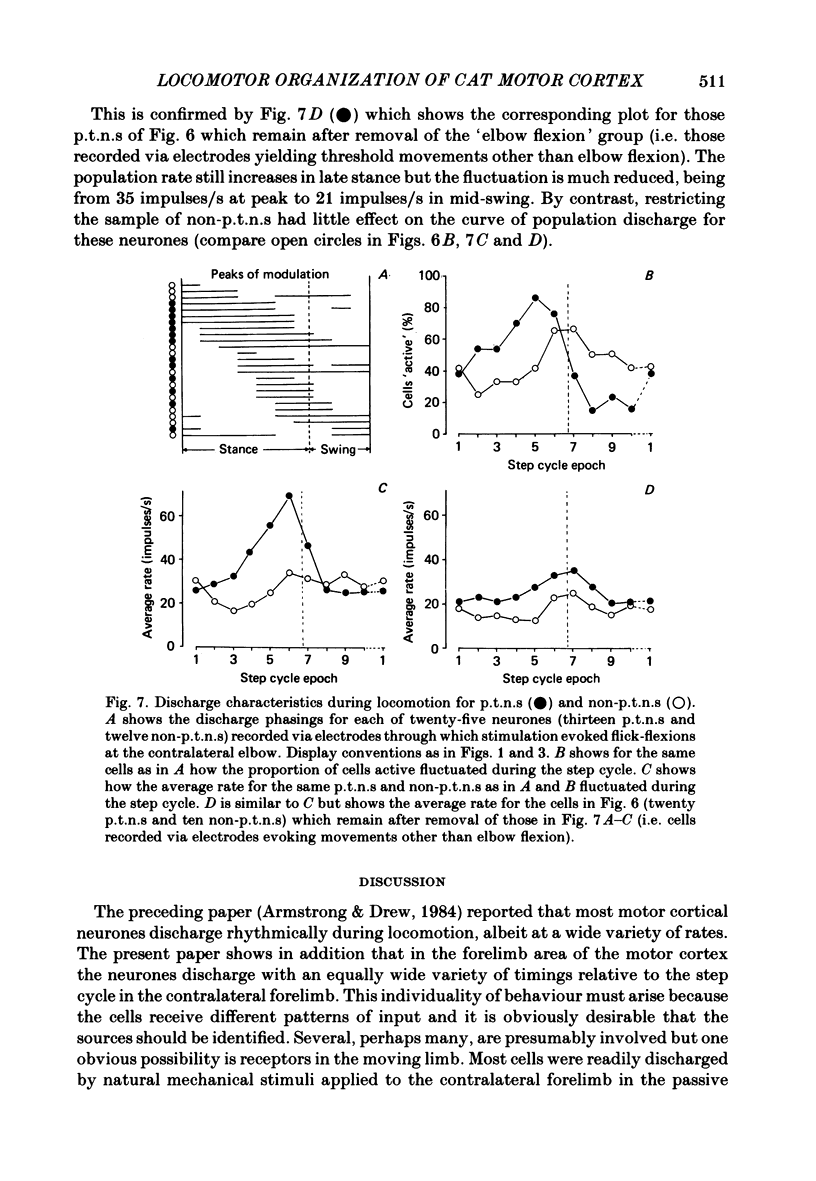
Abstract
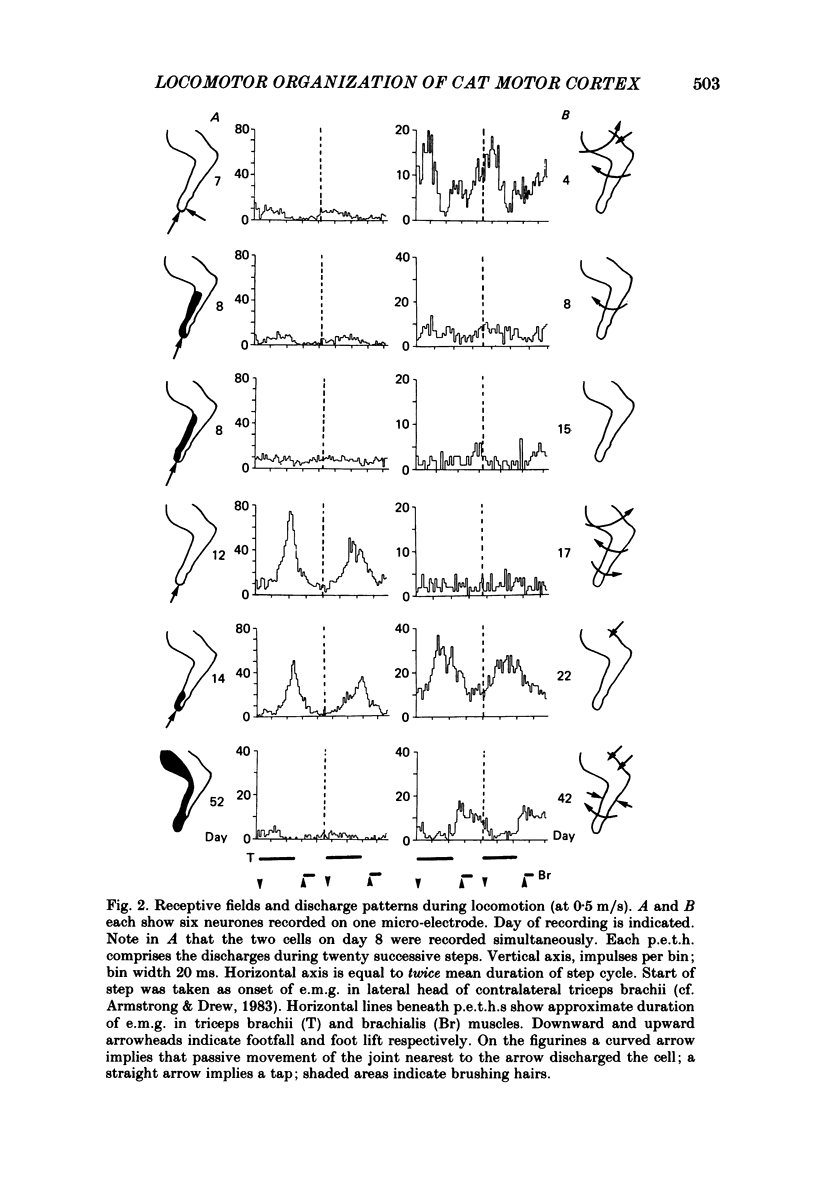
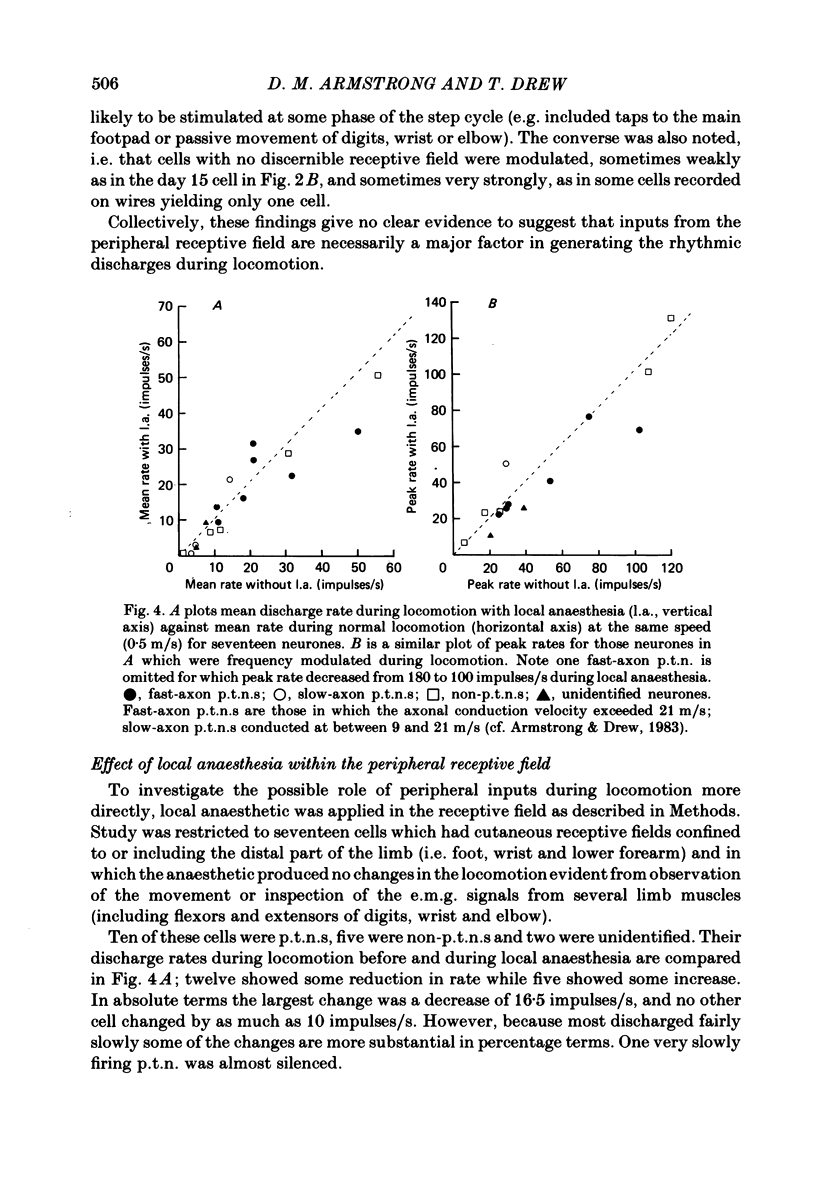
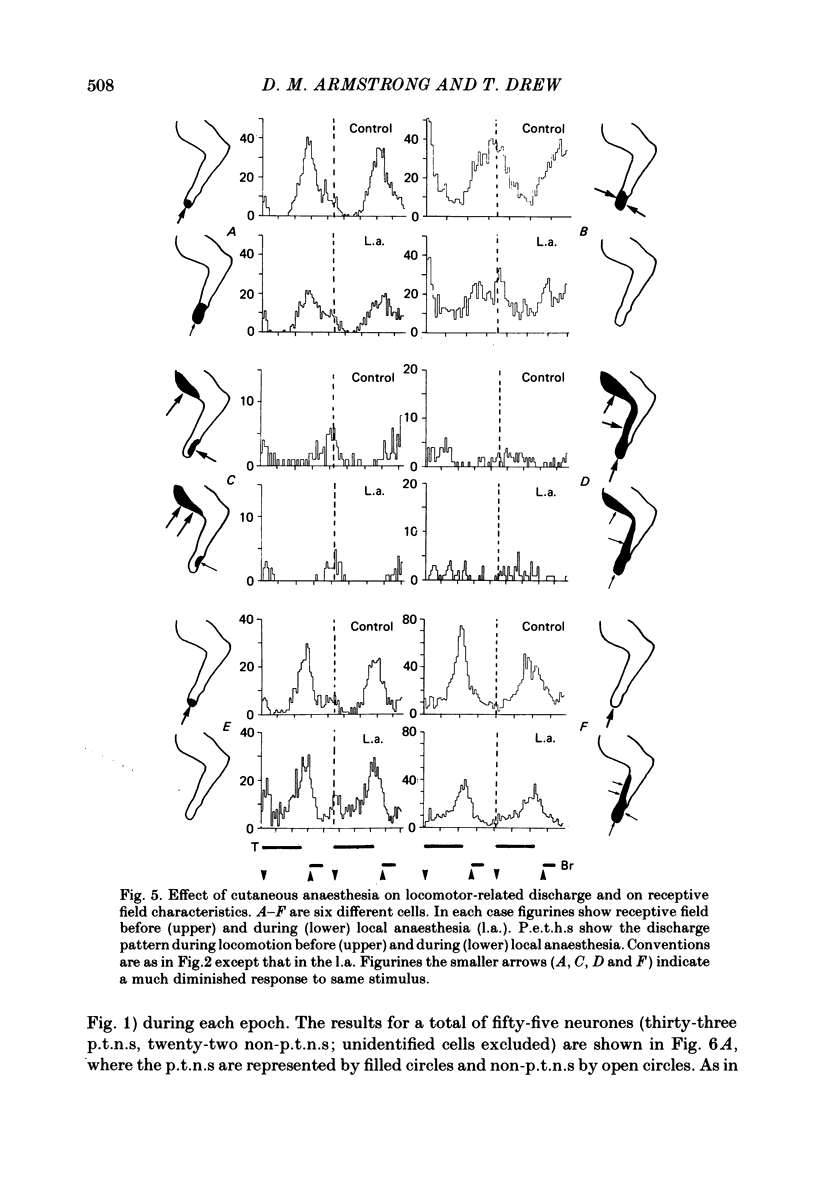
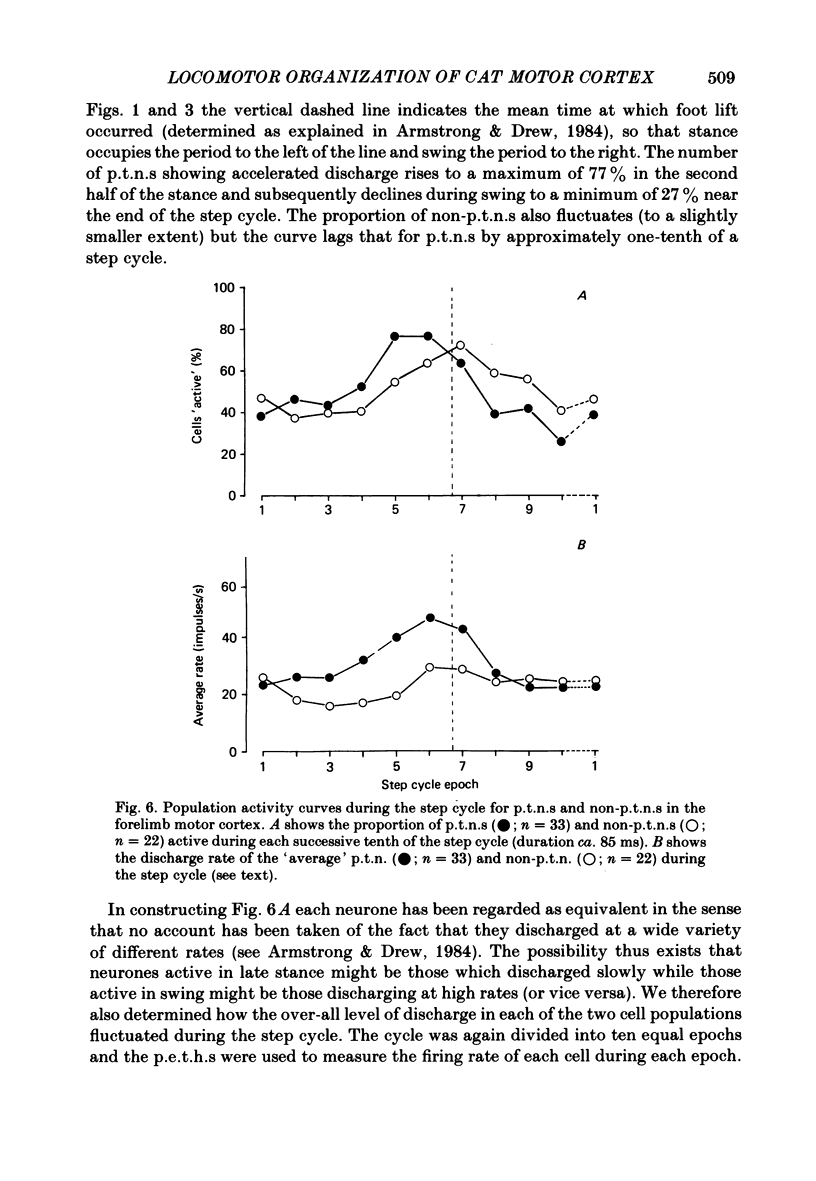
Discharge patterns of motor cortical neurones in cats walking steadily on a moving belt have been compared with other functional characteristics of the neurones. In forelimb motor cortex rhythmic discharges occurred in cells with peripheral receptive fields in all parts of the contralateral forelimb and also in cells with no discernible receptive field. Cells discharging at similar times during the step cycle often had very different receptive fields and cells with similar receptive fields (including neighbouring cells) could discharge at similar or at quite different times. In cells with a cutaneous receptive field including the forefoot the discharges during locomotion remained rhythmic (and their phasing relative to the step cycle was unchanged) when the response to mechanical stimulation in the receptive field was temporarily much reduced or abolished by local anaesthesia of the skin. The proportion of neurones showing accelerated firing during different parts of the step cycle fluctuated more for antidromically identified pyramidal tract neurones (p.t.n.s) than for non-p.t.n.s and was highest during the second half of stance in the contralateral forelimb and lowest during swing. When the neurones were subdivided according to the movement evoked by threshold electrical stimulation through the micro-electrode, p.t.n.s and non-p.t.n.s recorded by electrodes evoking elbow flexion showed a wide variety of discharge patterns. For p.t.n.s the discharge rate reached an average of 69 impulses/s during late stance and declined to an average of 26 impulses/s during swing.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antziferova L. I., Arshavsky Y. I., Orlovsky G. N., Pavlova G. A. Activity of neurons of cerebellar nuclei during fictitious scratch reflex in cat. I. Fastigial nucleus. Brain Res. 1980 Nov 3;200(2):239–248. doi: 10.1016/0006-8993(80)90916-6. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol. 1984 Jan;346:471–495. doi: 10.1113/jphysiol.1984.sp015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky Y. I., Berkinblit M. B., Fukson O. I., Gelfand I. M., Orlovsky G. N. Origin of modulation in neurones of the ventral spinocerebellar tract during locomotion. Brain Res. 1972 Aug 11;43(1):276–279. doi: 10.1016/0006-8993(72)90296-x. [DOI] [PubMed] [Google Scholar]
- Arshavsky Y. I., Orlovsky G. N., Pavlova G. A., Perret C. Activity of neurons of cerebellar nuclei during fictitious scratch reflex in the cat. II. Interpositus and lateral nuclei. Brain Res. 1980 Nov 3;200(2):249–258. doi: 10.1016/0006-8993(80)90917-8. [DOI] [PubMed] [Google Scholar]
- Asanuma H. Recent developments in the study of the columnar arrangement of neurons within the motor cortex. Physiol Rev. 1975 Apr;55(2):143–156. doi: 10.1152/physrev.1975.55.2.143. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Stoney S. D., Jr, Abzug C. Relationship between afferent input and motor outflow in cat motorsensory cortex. J Neurophysiol. 1968 Sep;31(5):670–681. doi: 10.1152/jn.1968.31.5.670. [DOI] [PubMed] [Google Scholar]
- Baker M. A., Tyner C. F., Towe A. L. Observations on single neurons recorded in the sigmoid gyri of awake, nonparalyzed cats. Exp Neurol. 1971 Sep;32(3):388–403. doi: 10.1016/0014-4886(71)90006-9. [DOI] [PubMed] [Google Scholar]
- Coulter J. D. Sensory transmission through lemniscal pathway during voluntary movement in the cat. J Neurophysiol. 1974 Sep;37(5):831–845. doi: 10.1152/jn.1974.37.5.831. [DOI] [PubMed] [Google Scholar]
- EVARTS E. V. RELATION OF DISCHARGE FREQUENCY TO CONDUCTION VELOCITY IN PYRAMIDAL TRACT NEURONS. J Neurophysiol. 1965 Mar;28:216–228. doi: 10.1152/jn.1965.28.2.216. [DOI] [PubMed] [Google Scholar]
- Eidelberg E., Yu J. Effects of corticospinal lesions upon treadmill locomotion by cats. Exp Brain Res. 1981;43(1):101–103. doi: 10.1007/BF00238815. [DOI] [PubMed] [Google Scholar]
- Evarts E. V. Contrasts between activity of precentral and postcentral neurons of cerebral cortex during movement in the monkey. Brain Res. 1972 May 12;40(1):25–31. doi: 10.1016/0006-8993(72)90101-1. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D., German D. C. Corticomotoneuronal connections of precentral cells detected by postspike averages of EMG activity in behaving monkeys. Brain Res. 1976 Sep 24;114(3):505–510. doi: 10.1016/0006-8993(76)90973-2. [DOI] [PubMed] [Google Scholar]
- Ghez C., Pisa M. Inhibition of afferent transmission in cuneate nucleus during voluntary movement in the cat. Brain Res. 1972 May 12;40(1):145–155. doi: 10.1016/0006-8993(72)90120-5. [DOI] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol Rev. 1975 Apr;55(2):247–304. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. Discharges of intracerebellar nuclear cells in monkeys. J Physiol. 1979 Dec;297(0):559–580. doi: 10.1113/jphysiol.1979.sp013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 1. Pyramidal effects on motoneurones. Exp Brain Res. 1976 Dec 22;26(5):509–519. doi: 10.1007/BF00238824. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Bak M. J., Duysens J. Long-term unit recording from somatosensory neurons in the spinal ganglia of the freely walking cat. Science. 1977 Sep 16;197(4309):1192–1194. doi: 10.1126/science.897663. [DOI] [PubMed] [Google Scholar]
- Nieoullon A., Rispal-Padel L. Somatotopic localization in cat motor cortex. Brain Res. 1976 Apr 9;105(3):405–422. doi: 10.1016/0006-8993(76)90590-4. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of rubrospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:99–112. doi: 10.1016/0006-8993(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of vestibulospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:85–98. doi: 10.1016/0006-8993(72)90007-8. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. The effect of different descending systems on flexor and extensor activity during locomotion. Brain Res. 1972 May 26;40(2):359–371. doi: 10.1016/0006-8993(72)90139-4. [DOI] [PubMed] [Google Scholar]
- Porter R., Lewis M. M. Relationship of neuronal discharges in the precentral gyrus of monkeys to the performance of arm movements. Brain Res. 1975 Nov 7;98(1):21–36. doi: 10.1016/0006-8993(75)90507-7. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol. 1976 Sep;39(5):1090–1104. doi: 10.1152/jn.1976.39.5.1090. [DOI] [PubMed] [Google Scholar]
- Viala G., Coston A., Buser P. Participation de cellules du cortex cérébelleux aux rythmes "locomoteurs" chez le lapin curarisé, en absence d'informations somatiques liées au mouvement. C R Acad Sci Hebd Seances Acad Sci D. 1970 Aug 17;271(7):688–691. [PubMed] [Google Scholar]


