Abstract
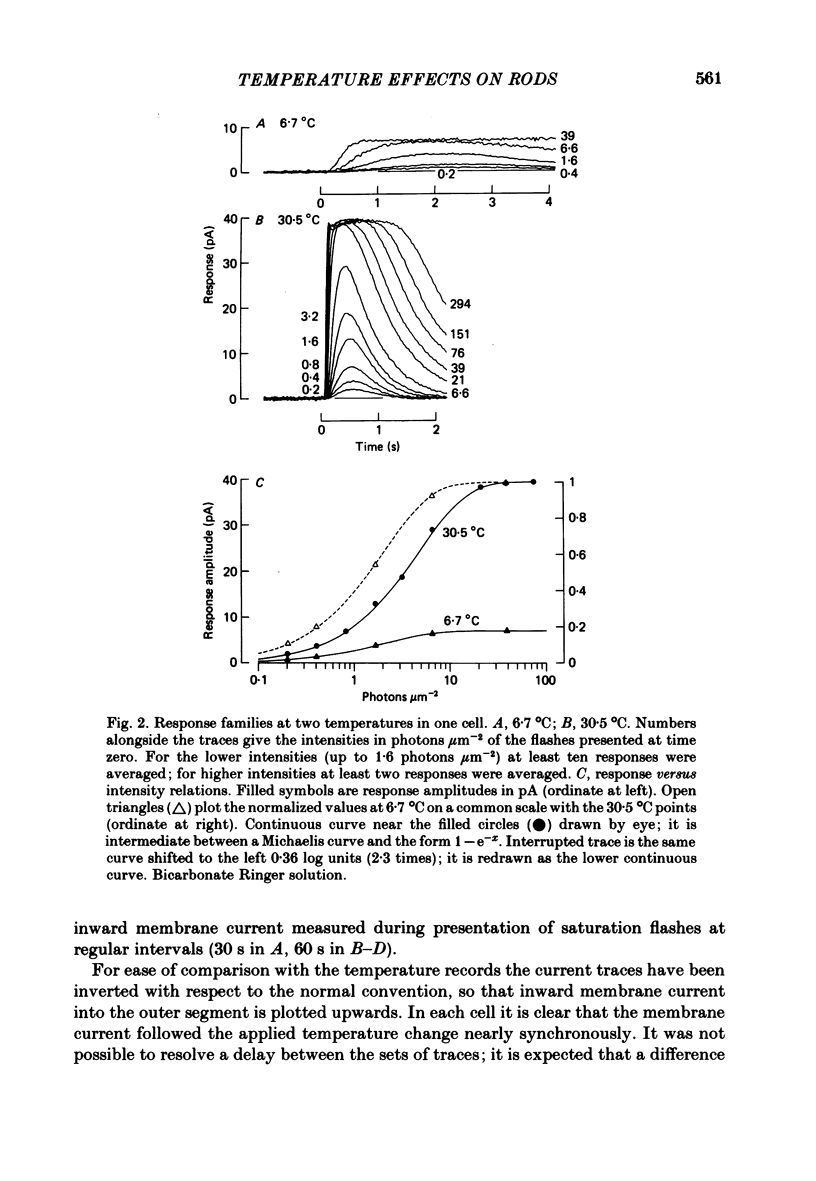
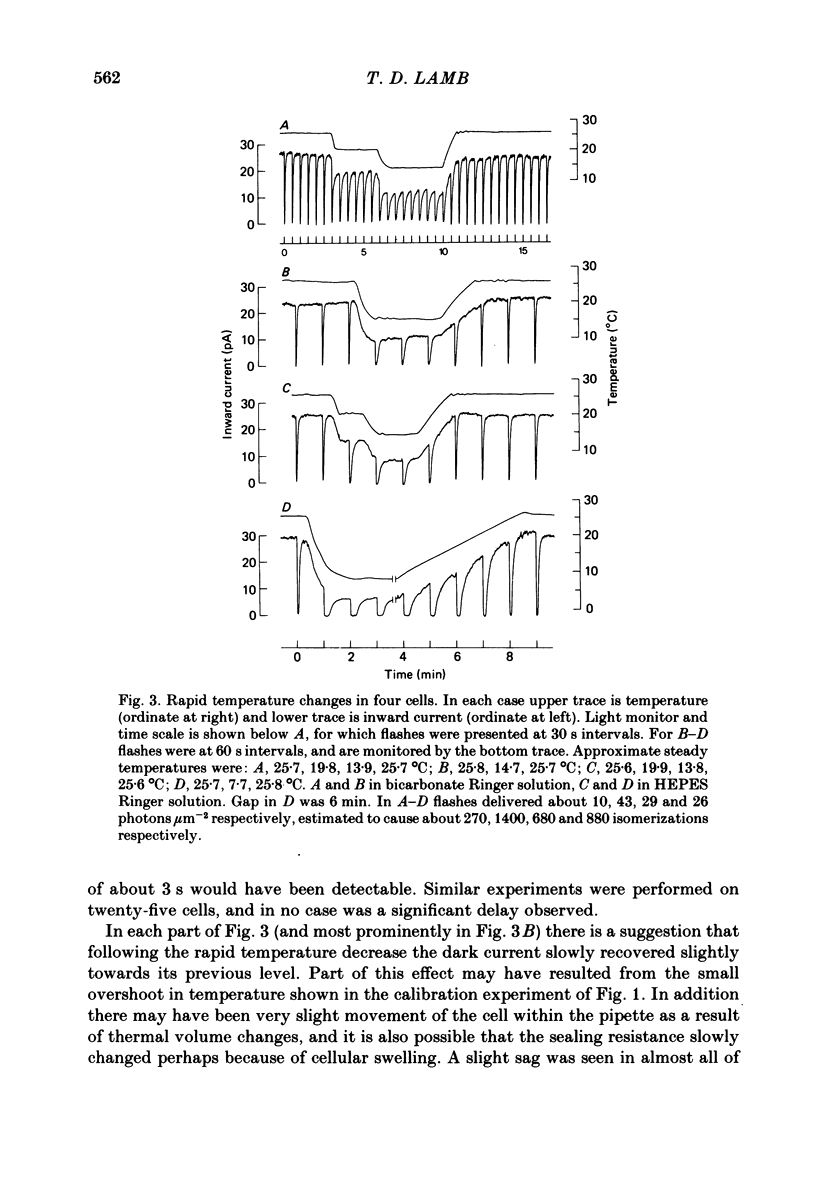
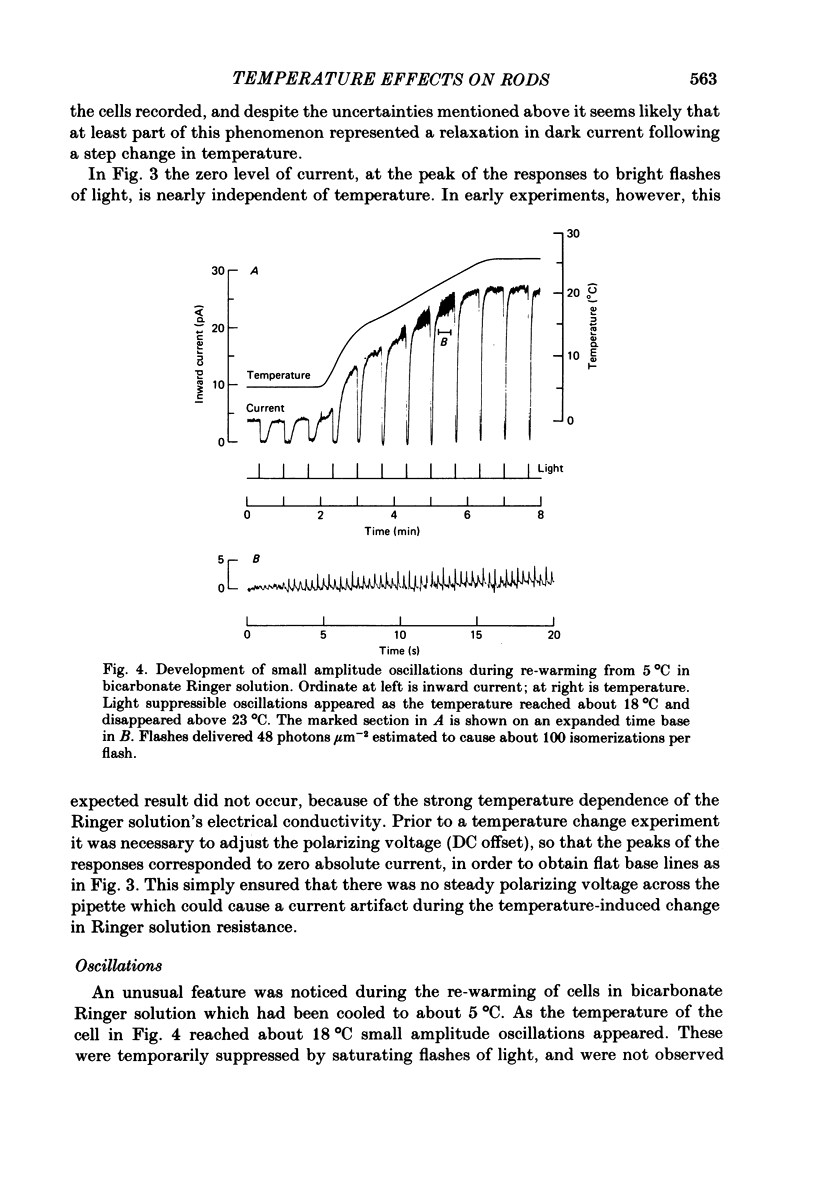
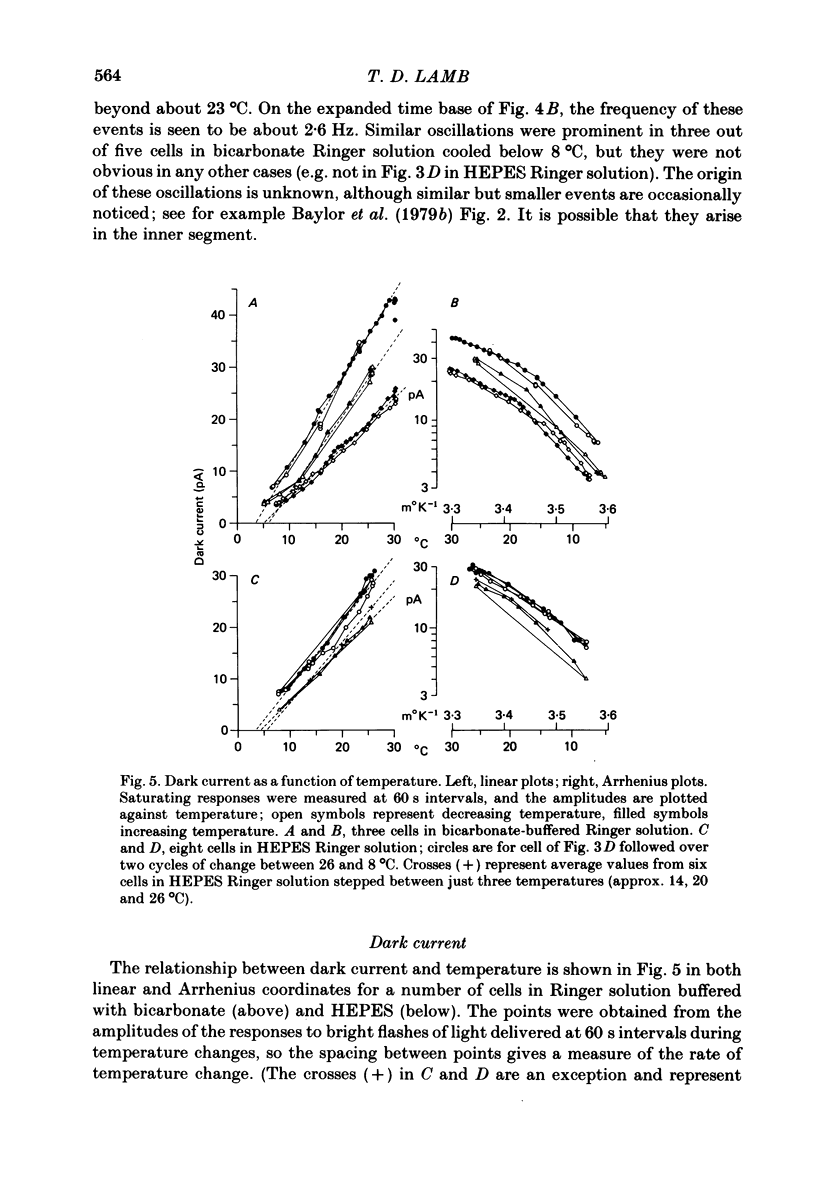
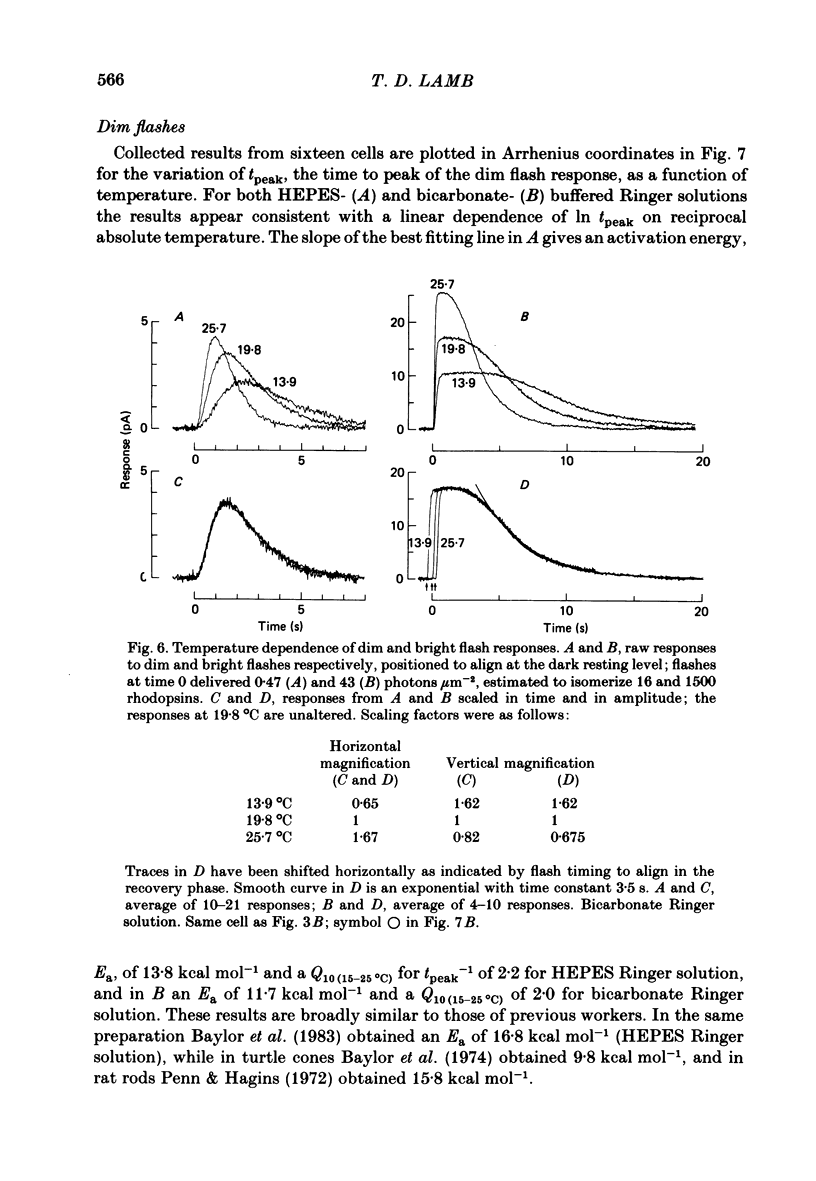
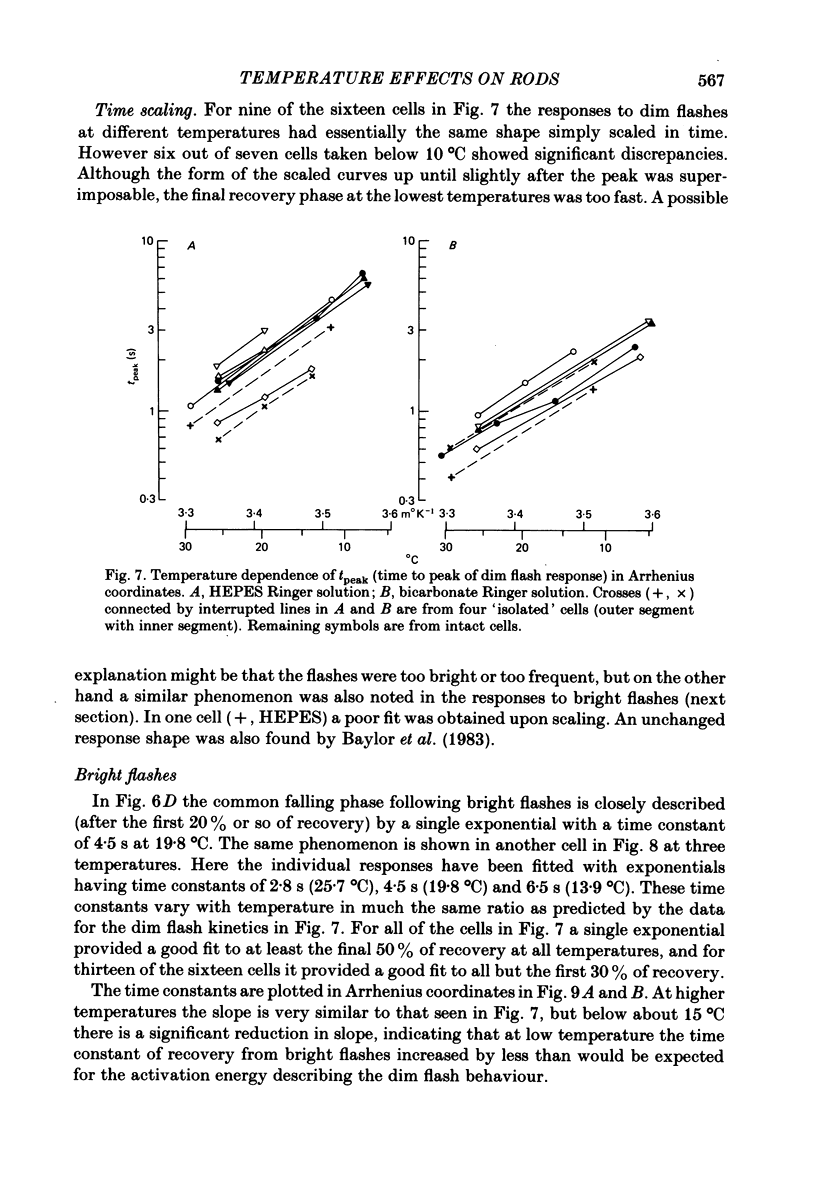
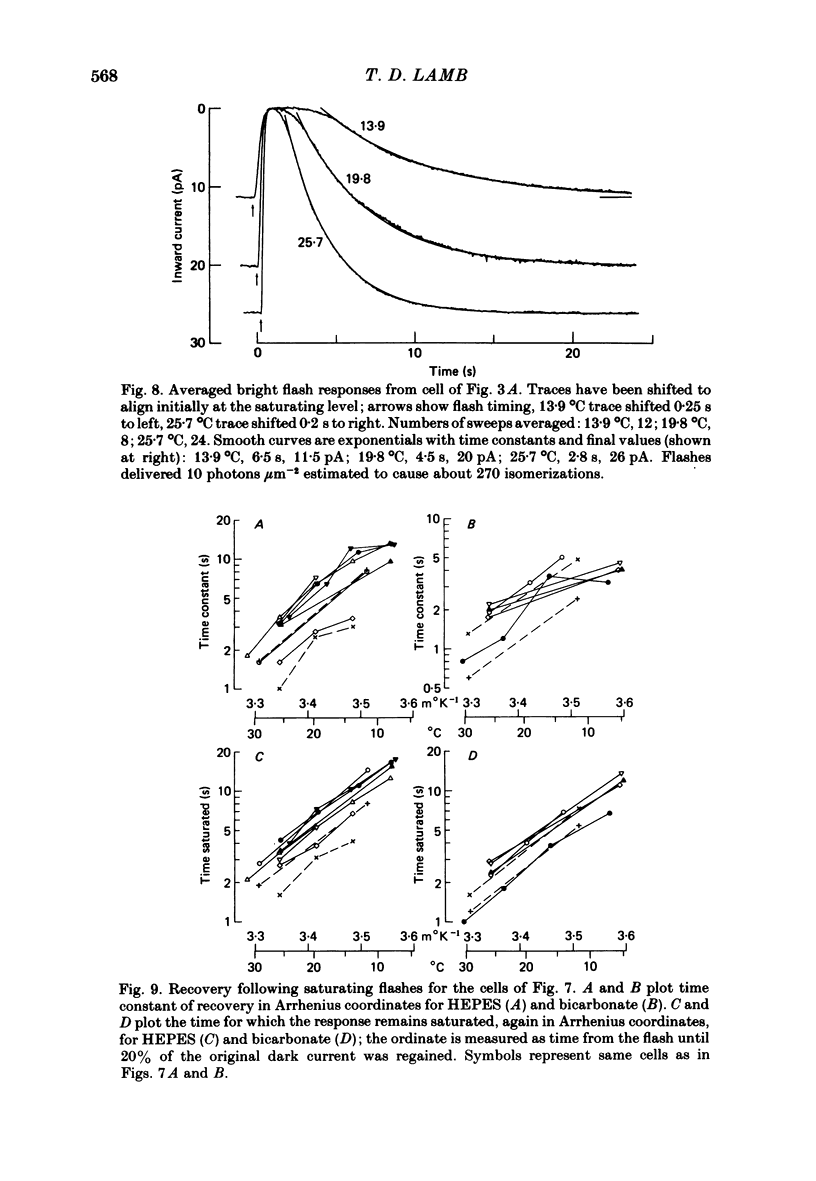
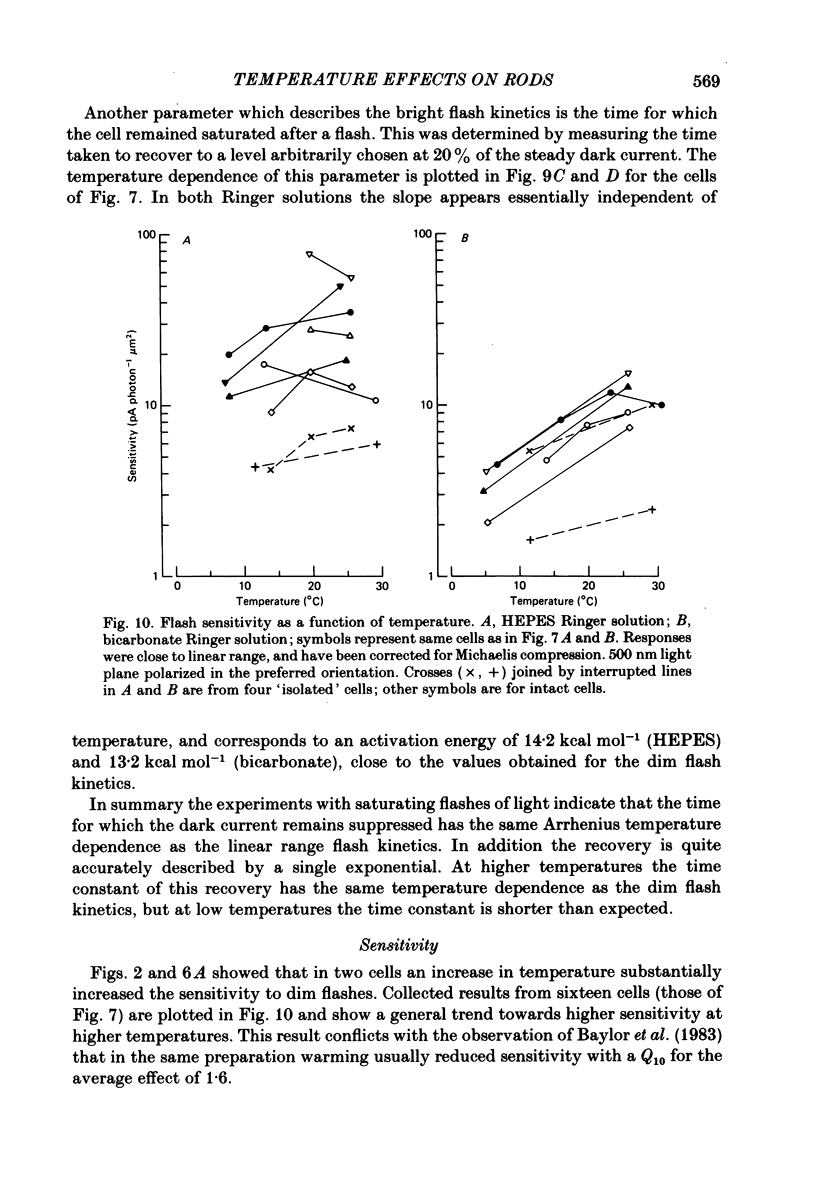
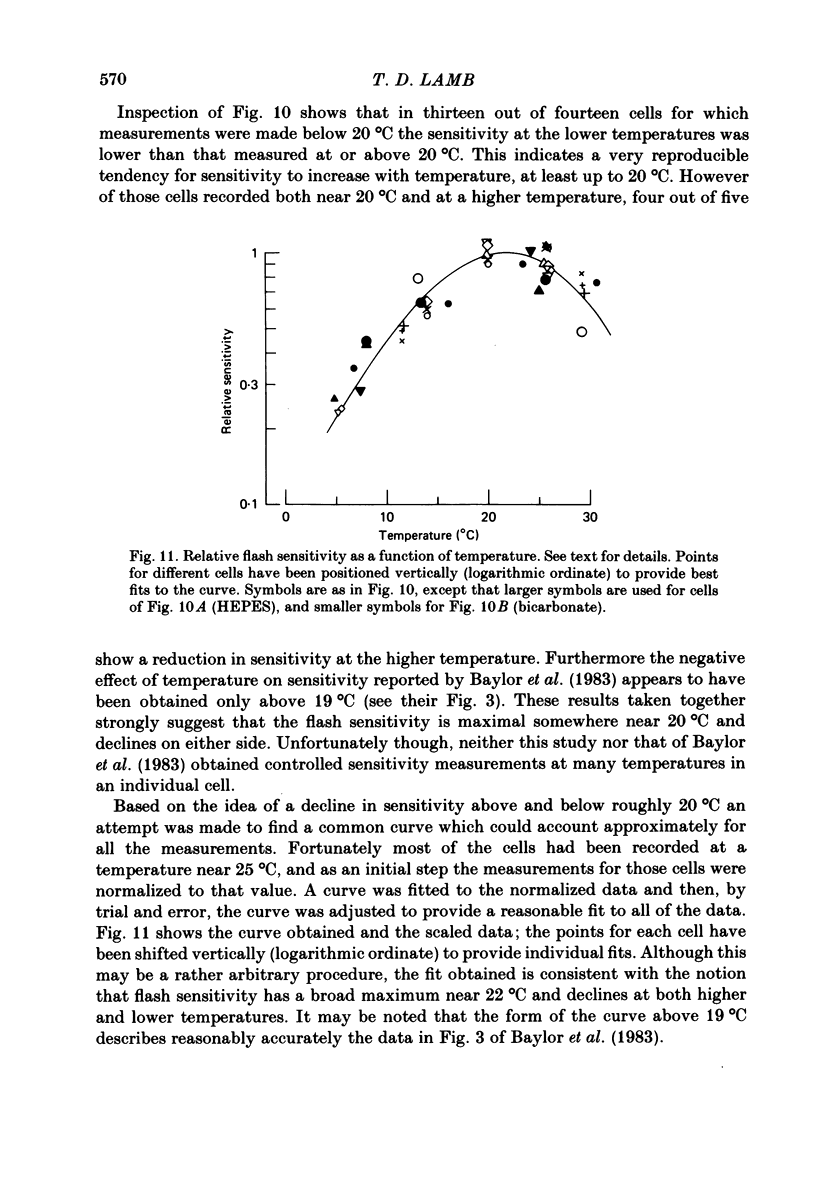
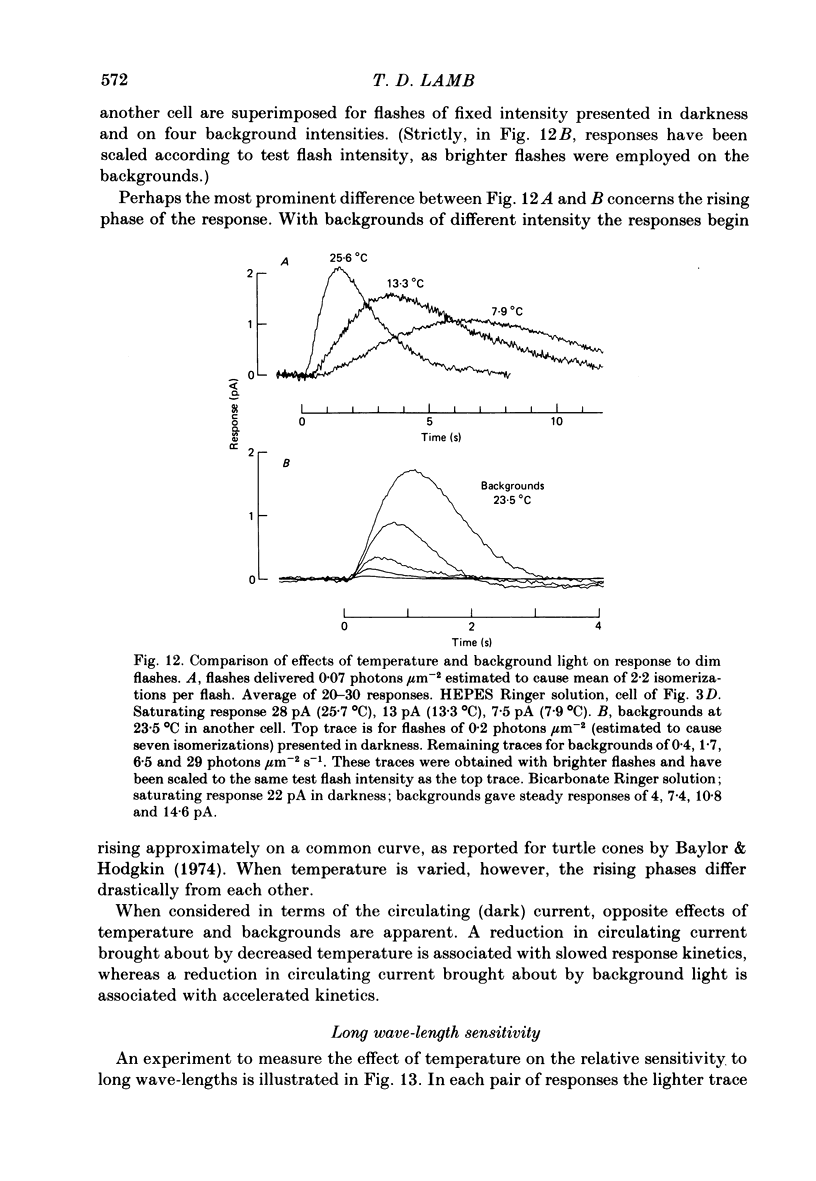
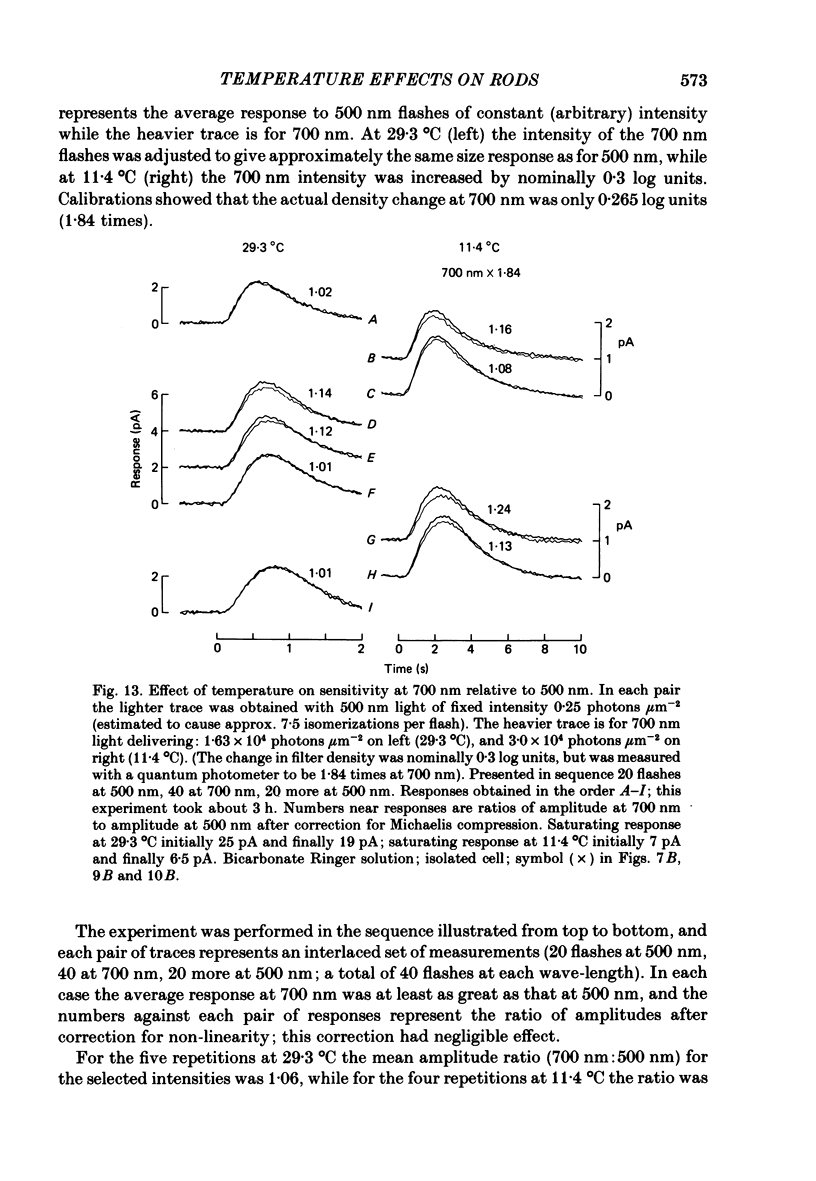
Rod current responses were measured over the range 5-30 degrees C. Following a rapid decrease in temperature the amplitude of the dark current decreased without detectable delay (less than 3 s). Over a period of several minutes the amplitude of the dark current sometimes relaxed slightly towards its previous value. The rapid change cannot be accounted for simply by altered activity of the sodium pump and instead indicates that the conductance of the outer segment in darkness changes with temperature. Over the range 10-30 degrees C the amplitude of the dark current increased approximately linearly with temperature, and the straight line of best fit extrapolated to zero current at about 5 degrees C. The few points available below 10 degrees C indicated that the relationship flattened out, but this could not be investigated properly. The kinetics of responses to dim flashes accelerated with a Q10 of about 2.2, and were well described by an Arrhenius plot with an activation energy of 13.8 kcal mol-1 (HEPES Ringer solution). The time course of recovery of dark current following a saturating flash showed a similar temperature dependence to that of the dim flash kinetics. A simple explanation of the previous two findings is that the delays determining the time course of responses to both dim and bright flashes are largely determined by the fluidity of the disk membrane. The sensitivity to dim flashes had a broad peak at about 22 degrees C, decreasing at both lower and higher temperatures. The relative sensitivity to long wave-length light increased slightly with temperature. The sensitivity at 700 nm relative to that at 500 nm increased by 0.225 log10 units (1.68 times) upon a temperature increase from 11.5 to 29.3 degrees C (from approximately -5.0 log10 units to approximately -4.8 log10 units). This change appears to be approximately what would be expected theoretically.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Temperature effects on the membrane current of retinal rods of the toad. J Physiol. 1983 Apr;337:723–734. doi: 10.1113/jphysiol.1983.sp014651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Pasino E., Torre V. Electrical responses of rods in the retina of Bufo marinus. J Physiol. 1977 May;267(1):17–51. doi: 10.1113/jphysiol.1977.sp011799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. H., Goldstein D. B. Effects of low concentrations of ethanol on the fluidity of spin-labeled erythrocyte and brain membranes. Mol Pharmacol. 1977 May;13(3):435–441. [PubMed] [Google Scholar]
- Cone R. A. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- DENTON E. J., PIRENNE M. H. The visual sensitivity of the toad Xenopus laevis. J Physiol. 1954 Jul 28;125(1):181–207. doi: 10.1113/jphysiol.1954.sp005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. S., Hagins W. A. Control of Ca2+ in rod outer segment disks by light and cyclic GMP. Nature. 1983 May 26;303(5915):344–348. doi: 10.1038/303344a0. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Schnetkamp P. P. Calcium metabolism in vertebrate photoreceptors. Cell Calcium. 1982 May;3(2):83–112. doi: 10.1016/0143-4160(82)90008-2. [DOI] [PubMed] [Google Scholar]
- LEWIS P. R. A theoretical interpretation of spectral sensitivity curves at long wavelengths. J Physiol. 1955 Oct 28;130(1):45–52. doi: 10.1113/jphysiol.1955.sp005391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Weiner H. L., Drzymala R. E. Lateral diffusion of visual pigment in rod disk membranes. Methods Enzymol. 1982;81:660–668. doi: 10.1016/s0076-6879(82)81091-4. [DOI] [PubMed] [Google Scholar]
- Orly J., Schramm M. Fatty acids as modulators of membrane functions: catecholamine-activated adenylate cyclase of the turkey erythrocyte. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3433–3437. doi: 10.1073/pnas.72.9.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebro R. A thermal component of excitation in the lateral eye of Limulus. J Physiol. 1966 Nov;187(2):417–425. doi: 10.1113/jphysiol.1966.sp008099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C. A., Bates R. G. Buffers for the physiological pH range: thermodynamic constants of four substituted aminoethanesulfonic acids from 5 to 50 degrees C. Anal Chem. 1976 Aug;48(9):1293–1296. doi: 10.1021/ac50003a010. [DOI] [PubMed] [Google Scholar]


