ABSTRACT
Background
Non-albicans Candida species (NACs) are commonly found in carious lesions, yet their specific role in caries progression remains unclear. Hence, we conducted an in vitro study to explore how NACs interactions with Streptococcus mutans affect cariogenicity.
Materials and Methods
Dual-species interkingdom biofilms were developed with S.mutans and six Candida species, C.albicans and the NACs: C.dubliniensis, C.glabrata, C.krusei, C.parapsilosis and C.tropicalis. Biofilm mass, viable cell counts, and pH were evaluated in mono- and dual-species biofilms. Quantitative RT-PCR was used to assess the expression of S.mutans genes associated with cariogenicity.
Results
Co-culturing S.mutans with either C.albicans, C.glabrata, or C.tropicalis significantly increased biofilm mass. While S.mutans numbers either increased or remained stable in dual-species biofilms, C.krusei, C.parapsilosis, and in particular C.tropicalis numbers significantly increased. All dual-species biofilms exhibited a pH below the critical demineralization level of enamel, akin to S.mutans mono-species biofilms. The expression of a battery of cariogenic genes in S.mutans was upregulated, particularly in dual-species biofilms with C.krusei and with C.tropicalis.
Conclusion
NACs influence the biofilm production and the cariogenic gene expression of S.mutans. The dual-species biofilm of S.mutans and NACs, particularly C.tropicalis, likely possess heightened cariogenic potential. Further research is warranted to unravel these intriguing interactions within interkingdom biofilms.
KEYWORDS: Biofilms, dental caries, Candida albicans, Candida tropicalis, Streptococcus mutans, non-albicans Candida species
Key messages
The coexistence of NACs, notably C. tropicalis, and S. mutans could amplify the cariogenicity of interkingdom biofilms.
Exploring the interplay between S. mutans and the mycobiome could help develop efficient strategies for caries prevention and management.
Introduction
Dental caries is a complex disease that arises when the oral environment becomes acidic, fostering the growth of acid-tolerant, cariogenic microorganisms [1]. The predominant cariogenic organism identified in this context is Streptococcus mutans, although more recently there have been calls for the inclusion of Candida species as a cariogen [2]. Numerous recent studies have also demonstrated the extremely high prevalence of Candida species in early childhood caries (ECC) and its severe variant (sECC) [3].
Indeed, Candida species are characterized by specific traits, such as acidogenicity, aciduricity, and the ability to form robust biofilms, that are the hallmarks of cariogenic organisms [4,5]. The foregoing microbiological data from children with ECC and sECC, and those from a number of other in vitro studies clearly indicate that Candida species in tandem with S. mutans may synergise cariogenicity [6].
For instance, the interactions between C. albicans and S. mutans are facilitated by the binding capacity of the GtfB protein of the latter with C. albicans cell wall mannoproteins, leading to enhanced glucan matrix synthesis resulting in profuse biofilm formation [7]. It is also known that this interkingdom symbiosis leads to the upregulation of genes associated with acid production and acid tolerance [8,9].
While historical data predominantly highlights the high prevalence of S. mutans and C. albicans in caries lesions, recent investigations, especially in children with ECC and sECC have revealed that non-albicans Candida species (NACs) are equally or more commonly found alongside these traditional cariogenic agents [10,11]. For instance, de Carvalho et al. found that C. albicans is the most prevalent species in ECC, followed by C. tropicalis and C. krusei [11]. More recently, Fakhruddin KS et al. discovered, in a UAE cohort, that NACs, specifically C. tropicalis and C. krusei, were more prevalent than C. albicans in sECC [3,10]. The reasons behind this cohabitation of S. mutans and NAC species in carious lesions remain obscure. However, it is tempting to speculate that differences in cell wall mannoproteins between NACs and C. albicans may play a role [12–15]. For instance, C. krusei has been found to have less abundant mannan compared to C. albicans [16], and C. glabrata possesses short and lightly branched cell wall mannans [15]. Furthermore, studies have shown that the GtfB enzyme produced by S. mutans can avidly bind to the cell walls of various Candida species [8]. These variations in cell wall composition and binding properties in NACs may influence the interactions between such dual-species, interkingdom biofilms.
Therefore, the main aim of this study was to characterize the cariogenic attributes of interkingdom, dual-species biofilms of NACs and S. mutans in vitro, in terms of the total biofilm mass, viable cell counts, and pH alterations. We also took the opportunity to evaluate the expression of cariogenic virulence genes in S. mutans during such interactions.
Materials and methods
Microorganism strains and growth conditions
Type culture strains of Streptococcus mutans UA159, Candida albicans SC5314, Candida dubliniensis NCPF3949, Candida glabrata (Nakaseomyces glabrata) ATCC2001, Candida tropicalis ATCC750, Candida parapsilosis ATCC90018 and Candida krusei (Pichia kudriavzevii) ATCC6258 were used in this study. Streptococcus mutans and Candida spp. were separately grown in BHI broth at 37°C and 30°C, respectively, with shaking (at 240 rpm) until the cultures reached the log phase (OD600 nm = 0.4–0.6; approximately 4 hours).
Biofilm formation and gram staining
For use in mono-species biofilm formation studies, log phase cultures of S. mutans and Candida spp. were adjusted to OD600 nm of 0.4 (approximately 108 CFU/ml) and OD600 nm of 0.1 (approximately 106 CFU/ml), respectively. The cell pellets of each microorganism were then collected from 1 ml of culture by centrifugation and re-suspended in 500 µl of BHI broth supplemented with 5% sucrose before transferring to separate wells in a 24-well plate (a polystyrene plate, Corning Costar, Kennebunk, ME, USA). The plate was incubated for 36 hours at 37°C with 5% CO2 to form biofilm, without changing the media.
Dual-species biofilms were created using the same approach employed for mono-species biofilm, with microorganisms being combined at a ratio of 108 CFU/ml for S. mutans and 106 CFU/ml for Candida spp., mimicking the proportions typically observed in the saliva samples of children with ECC [8]. After 36 hours of incubation, the biofilms were washed three times with sterile phosphate-buffered saline (0.01 M PBS, pH 7.4) to remove non-adherent cells. Both mono- and dual-species biofilms were gram-stained, and representative photomicrographs were obtained.
Determination of biofilm mass using crystal violet assay
The 36-hour biofilm was fixed with 500 µl of 95% ethyl alcohol for 20 minutes at room temperature and then stained with 500 µl of 0.1% (w/v) crystal violet (CV) for 15 minutes. After that, the stained biofilm was washed three times with distilled water and let dry. The biofilm then was de-stained with 33% acetic acid. The optical density of the de-staining solution was measured at 520 nm [17]. The culture medium without microorganisms was used as a blank. The experiments were performed five times independently. Biofilm mass was calculated using the following formula:
Determination of cell viability by colony counting
The 36-hour biofilm was scraped and re-suspended in sterile PBS. The cell clumps were disrupted by an ultrasonic homogenizer with an amplitude of 25% for 30 seconds twice [18]. The homogenized suspension was serially diluted and plated on 1) Mitis-Salivarius agar (MS agar: selective media for streptococci) and 2) Sabouraud dextrose agar (SDA) for Candida species. MS agar plates were incubated at 37°C with 5% CO2 whereas SDA plates were incubated at 30°C for approximately 36 hours [18]. After incubation, the number of colony-forming unit (CFU) was determined. The experiments were performed five times independently. The number of microorganisms was presented as Log CFU/ml.
Determination of biofilm pH
In our preliminary study, no difference in pH values was observed between the biofilm and the culture medium. Therefore, the pH of the biofilm was determined by assessing the pH of the culture medium. The pH was measured by a pH meter (LAQUAtwin, HORIBA, Singapore) at 36 hours. The experiments were repeated on five independent occasions.
Moreover, to investigate the dynamic change, the pH was measured at the following time points: the beginning of the experiment (0 min), 15 min, 30 min, 45 min, 1 h, 2 h, 3 h, 4 h, 8 h, 12 h, 24 h and 36 h. These experiments were repeated on three different occasions.
Determination of gene expression of Streptococcus mutans associated with its attributes of cariogenicity
RNA extraction and purification
A previously described protocol was employed with minor modifications to determine the gene expression of Streptococcus mutans that are known to be associated with their attributes of cariogenicity [19]. These genes were chosen based on prior research that highlighted the upregulation of S. mutans virulence genes when co-cultured with C. albicans [8,9]. Table 1 illustrates the S. mutans genes so chosen and their primer sequences.
Table 1.
List of primer sequences of cariogenic virulence genes of Streptococcus mutans.
| Genes | Primer sequences | Descriptions | Ref. |
|---|---|---|---|
| EPS synthesis and glucan binding | |||
| gtfB | F: 5’- CACTATCGGCGGTTACGAAT-3’ R: 5’- CAATTTGGAGCAAGTCAGCA-3’ |
Glucosyltransferase GTF-I | Wang et al. [20] |
| gtfC | F: 5’- GATGCTGCAAACTTCGAACA −3’ R: 5’- TATTGACGCTGCGTTTCTTG −3’ |
Glucosyltransferase GTF-SI | Wang et al. [20] |
| gtfD | F: 5’- TTGACGGTGTTCGTGTTGAT −3’ R: 5’- AAAGCGATAGGCGCAGTTTA −3’ |
Glucosyltransferase-S | Wang et al. [20] |
| gbpB | F: 5’- CGTGTTTCGGCTATTCGTGAAG −3’ R: 5’- TGCTGCTTGATTTTCTTGTTGC −3’ |
Glucan-binding protein B | Wen et al. [21] |
| Sugar utilization | |||
| scrA | F: 5’- GATTGCCCTCAGCAGTTGACAT −3’ R: 5’- GCTGGGAAACTTTGATGGAGAC −3’ |
PTS system sucrose-specific transporter subunit IIABC | Li et al. [22] |
| scrB | F: 5’- ACAGCCTGTCCTGATTTATAGTC −3’ R: 5’- CTGGTAACCCAATCCATGAGAC −3’ |
Sucrose-6-phosphate hydrolase | Li et al. [22] |
| ldh | F: 5’- AAAAACCAGGCGAAACTCGC −3’ R: 5’- CTGAACGCGCATCAACATCA −3’ |
Lactate dehydrogenase | Wang et al. [20] |
| pdhA | F: 5’- ATGCCAAACTATAAAGATTTAC −3’ R: 5’- TCTTGGGCTTCAATATCT −3’ |
Pyruvate dehydrogenase, TPP-dependent E1 component alpha-subunit | He et al. [9] |
| pflA | F: 5’- ACGACCTTGGATACCTGTGC −3’ R: 5’- AAGACATGGCGAATCCAAAC-3’ |
Pyruvate formate-lyase | This study |
| Aciduricity | |||
| atpD | F: 5’- TGTTGATGGTCTGGGTGAAA −3’ R: 5’- TTTGACGGTCTCCGATAACC −3’ |
F1F0-ATP synthase subunit beta | Bezerra et al. [23] |
| fabM | F: 5’- ACTGATTAATGCCAATGGGAAAGTC −3’ R: 5’- TGCGAACAAGAGATTGTACATCATC −3’ |
Enoyl-CoA hydratase | Bezerra et al.[23] |
| Signal transduction system | |||
| ciaH | F: 5’- CGTCATCAATAATGTCAATGCCTTC −3’ R: 5’- TACCTTAACTGTCACTGTCCGATAC −3’ |
Histidine kinase sensor CiaH | Hu et al. 24] |
| ciaR | F: 5’- GAAGCAGAGTGGCGTTTATG −3’ R: 5’- TGTCATCCAAACCTTCCTTAGC −3’ |
Response regulator CiaR | He et al. [9] |
| Housekeeping genes | |||
| rpoB | F: 5’- GCAGTCAAGGGGTGGAAATCG −3’ R: 5’- TGGACGGCTTGTTGCAGGAATAC −3’ |
DNA-dependent RNA polymerase beta-subunit | Park et al. [25] |
The 36-hour biofilms were scraped from 24-well plates and kept in RNAlater (Invitrogen, ThermoFisher) at 4°C until use. The biofilms were suspended in cold PBS, sonicated by an ultrasonic homogenizer (Sonics, USA) at an amplitude of 25% for 30 seconds and then centrifuged at 5,500 g, 4°C, for 10 minutes. This sonication-washing step was repeated twice to break cell clumps.
After the final washing step, the cells were re-suspended in 0.25 ml of NAES buffer (50 mm sodium acetate buffer, 10 mm EDTA and 1% SDS, pH 5.0) and transferred to new 2.0 ml microcentrifuge tubes. Glass beads (0.1 mm diameter) were added into the tubes until the final volume reached 0.5 ml, and an equal volume of acid phenol/chloroform (5:1, pH 4.5) was added and mixed by vortexing with maximum speed at 40 seconds to break the cells. This vortexing step was repeated twice and then the cell extract was centrifuged at 14,000 g, 25°C for 15 minutes. To isolate the nucleic acid, the aqueous layer was collected and mixed with an equal volume of acid phenol/chloroform (5:1, pH 4.5) for 10 seconds and centrifuged at 14,000 g, 4°C for 15 minutes. The aqueous layer was collected and mixed with an equal volume of chloroform/isoamyl alcohol (24:1) and centrifuged at 14,000 g, 4°C for 5 minutes. The aqueous phase was transferred to a new 1.5 ml microcentrifuge tube. Nucleic acid (RNA/DNA) was precipitated using a 1/10 volume of 3 M sodium acetate (pH 5.0) and 1 volume of cold isopropanol and stored at −20°C for at least 30 minutes. The pellets were collected by centrifugation at 14,000 g, 4°C for 15 minutes and washed with 0.5 ml of cold 75% (v/v) ethanol three times followed by 0.5 ml of 99% cold ethanol. The pellets were dried at 65°C for 3 minutes and was dissolved in 20 µl of sterile distilled-DEPC water and stored in −80°C until use.
The samples were treated with DNase I (Thermo Fisher Scientific, USA) for 30 minutes at 37°C to remove DNA and purified using ethanol precipitation to remove the DNase. The RNA concentration was quantified using the NanoDrop™ 2000/2000c spectrophotometers (Thermo Fisher Scientific, USA).
Quantitative reverse transcription PCR (qRT-PCR)
The bacterial mRNA was converted to cDNA by using Random Hexamer primer (Macrogen, Korea) and ImProm-II™ Reverse Transcriptase (Promega, USA) according to the manufacturer’s instructions. Subsequently, quantitative real-time PCR (qPCR) with Luna Universal qPCR master mix (New England Biolabs, USA) was performed using primers shown in Table 1. The sequences of pfl primers were designed in this study using Primer3Plus software (access: https://primer3plus.com).
A reaction mixture was prepared by mixing 5 µl of qPCR master mix, 0.25 µl of each (10 µM) primer, 3 µl of cDNA template and sterile MilliQ water up to 10 µl. The qRT-PCR was performed using the following cycling parameters: 1 cycle of initial denaturing at 95°C for 10 minutes and amplified for 40 cycles with denaturing at 95°C for 15 seconds, followed by annealing and extension at 60°C for 30 seconds. Melting curve analysis was subsequently performed from 60–95°C. The rpoB gene was used as a housekeeping gene [25]. Levels of gene expression were calculated using the double delta CT method. The experiments were performed three times independently. Finally, the expression levels were transformed to log2 using GraphPad Prism version 10.0.3 for Windows (GraphPad Software, Boston, Massachusetts, USA) and the results were converted into a heatmap (Figure 5).
Figure 5.
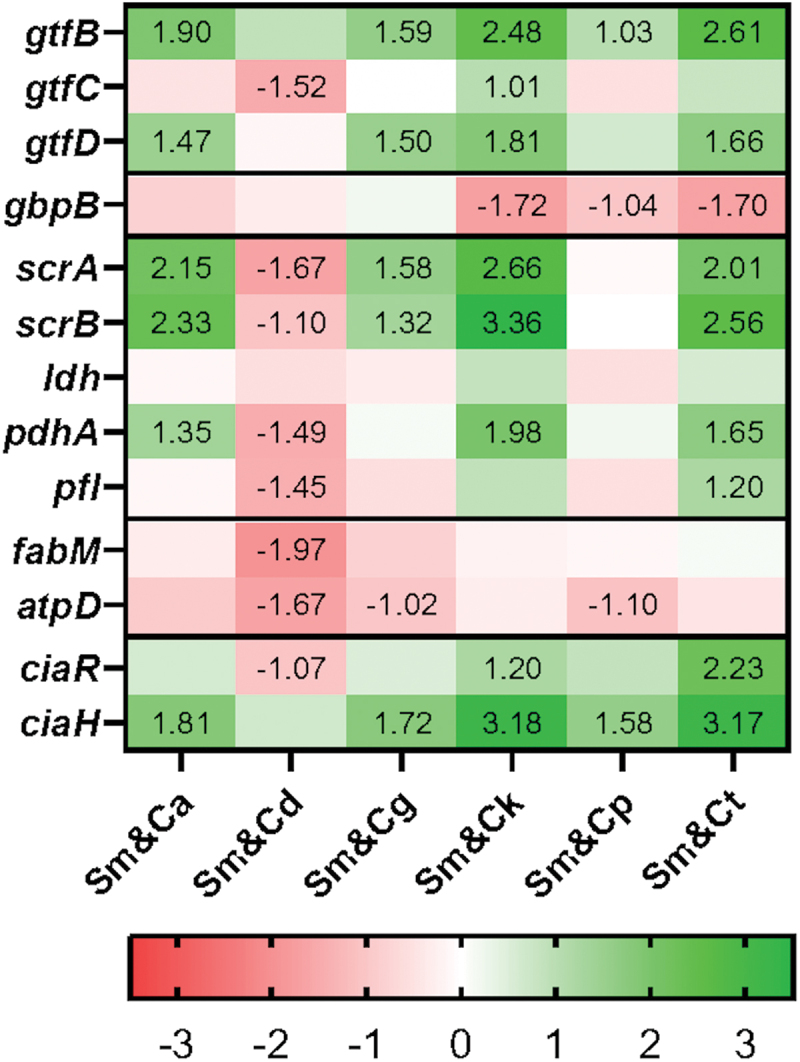
The expression of genes associated with S. mutans cariogenicity in dual-species biofilms (presented as a log2-fold change relative to S. mutans mono-species biofilms). A log2-fold change over 1 was defined as upregulation, while a value less than − 1 was considered as downregulation (label value). The experiments were performed on three separate occasions.
Statistical analysis
All data were analysed using GraphPad Prism version 10.0.3 for Windows (GraphPad Software, Boston, Massachusetts, USA). The normality test was done by using the Shapiro-Wilk test. The differences in biofilm mass and the colony numbers of S. mutans and Candida species between mono- and the respective dual-species biofilms were evaluated with the Mann-Whitney U test (not normal distribution) or t-test (normal distribution). The statistical significance level was set at α = 0.05.
Results
Mass determination and gram-staining of S. mutans, and NAC species in mono- and dual-species interkingdom biofilms
The biofilm mass of mono- and dual-species was evaluated relative to that of mono-species (as described in materials and methods). The dual-species biofilm (ds/ss) of S. mutans (Sm) with C. albicans (Ca), C. glabrata (Cg) and C. tropicalis (Ct) showed significantly higher biofilm mass than the sum of their mono-species biofilms (p < 0.05, Figure 1a). The biomass of the other three dual-species biofilms of C. dubliniensis (Cd), C. krusei (Ck), and C. parapsilosis (Cp) also appeared higher than the mono-species biofilm, but the difference was not statistically significant (p > 0.05). Finally, no significant difference was noted between the biofilm mass of Sm&Ca (ds/ss), and that of all Sm&NACs (ds/ss).
Figure 1.
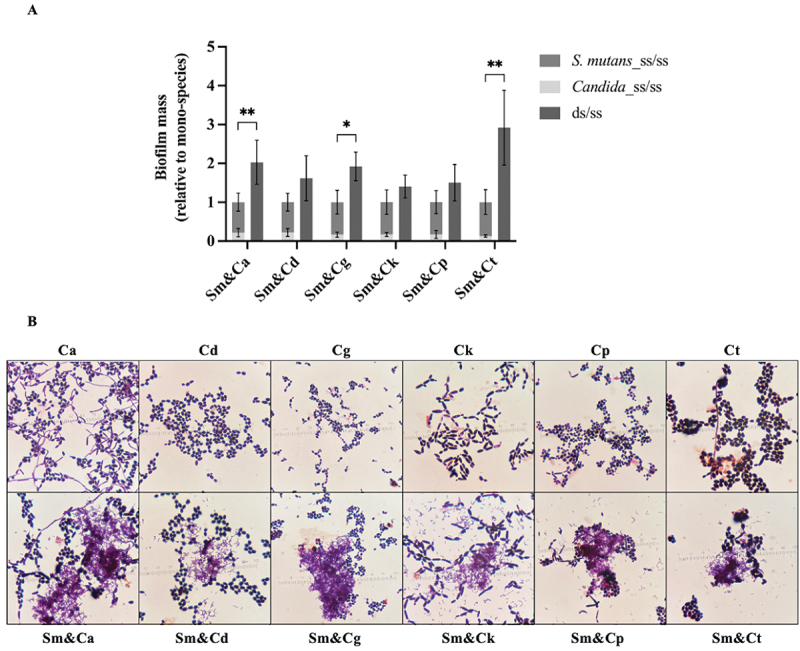
The mass of S. mutans and NAC species, along with the gram-staining of Candida spp., in mono- and dual-species interkingdom biofilms. (a) Biofilm mass of mono- and dual-species relative to that of the mono-species counterparts. The data are means and standard deviations of five separate experiments (n = 5). Asterisks denote statistical significance (*p < 0.05 and **p < 0.01). (b) Gram staining of Candida species in mono- (upper row) and dual-species biofilms with S. mutans (lower row). Filamentation and yeast forms were observed in C. albicans mono- and dual-species biofilms, while NACs predominantly remained in yeast form, except for C. tropicalis, which formed a small number of hyphae. Sm: S. mutans, Ca: C. albicans, Cd: C. dubliniensis, Cg: C. glabrata, Ck: C. krusei, Cp: C. parapsilosis, Ct: C. tropicalis.
The microscopic morphology and structure of the microorganisms in the mono- and dual-species biofilm after 36 hours of incubation were examined following Gram staining (Figure 1b). It was evident that C. albicans exhibited growth in both yeast and hyphae forms within the mono- and dual-species biofilms. In contrast, the majority of the NACs were in yeast phase, except C. tropicalis that exhibited a sparse amount of hyphal growth.
Quantitation of cell numbers of S. mutans, and NAC species in mono- and dual-species biofilms
When the viable cell numbers of S. mutans in mono-species biofilms were compared with those in dual-species biofilms, the streptococcal growth in C. albicans, C. glabrata and C. parapsilosis dual-species biofilms was noted to be significantly higher than in the respective mono-species counterpart biofilms (Figure 2a).
Figure 2.
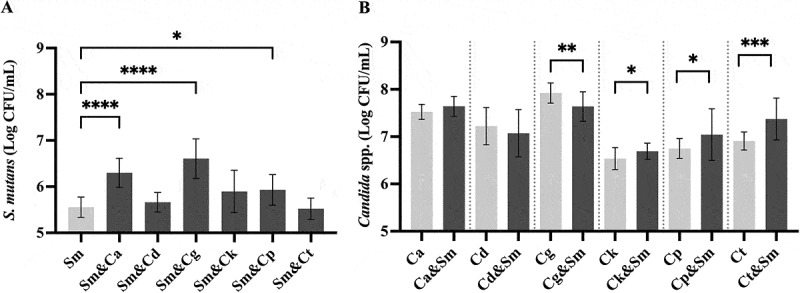
A comparison of the numbers of viable S. mutans and NAC species cells in mono- and dual-species biofilms. The data are mean and standard deviations of five separate experiments (n = 5). The viable (a) S. mutans and (b) Candida species cell numbers in mono- (light color) and dual-species biofilms (dark color). Asterisks denote statistical significance; *p < 0.05, **p < 0.01, ***p ≤ 0.001 and ****p < 0.0001. Sm: S. mutans, Ca: C. albicans, Cd: C. dubliniensis, Cg: C. glabrata, Ck: C. krusei, Cp: C. parapsilosis, Ct: C. tropicalis.
Conversely, when assessing the viable cell numbers of Candida spp. in mono-species versus dual-species biofilms, C. krusei, C. parapsilosis, and C. tropicalis displayed significantly higher cell counts in dual-species growth compared to their counterpart mono-species biofilms (Figure 2b). One exception was the growth of C. glabrata which was significantly impeded in dual-species biofilms, in contrast to its mono-species growth.
The acidogenic potential of S. mutans, and NAC species in mono- and dual-species biofilms
The critical pH of 5.5 required for enamel demineralization was used as a surrogate marker indicating the cariogenic potential of biofilms. Typically, the average pH of the mono-species biofilms of all Candida species surpassed this critical pH of 5.5. In contrast, the pH of all dual-species biofilms dropped below this threshold, mirroring that of S. mutans. This suggests that the growth of S. mutans and yeast biofilms is associated with acid production irrespective of the presence of the yeasts (Figure 3).
Figure 3.
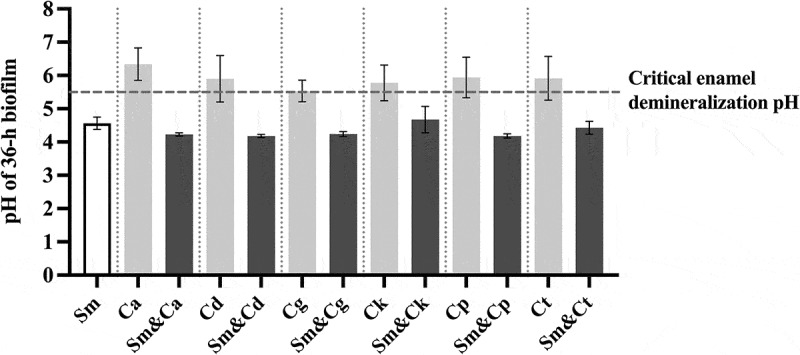
The pH values of mono- and dual-species biofilms at 36 hours. The data are mean and standard deviations of five separate experiments (n = 5). The white bar represents S. mutans, the light-colored bars represent Candida spp. in mono-species biofilms, and the dark-colored bars represent S. mutans and Candida spp. in dual-species biofilms. The dotted line indicates the critical enamel demineralization pH of 5.5. Sm: S. mutans, Ca: C. albicans, Cd: C. dubliniensis, Cg: C. glabrata, Ck: C. krusei, Cp: C. parapsilosis, Ct: C. tropicalis.
Aside from the final pH of the biofilm, after 36 hours, we also examined the temporal increase of the biofilm’s acidogenicity over the same duration (Figure 4). These pH dynamics echoed the earlier data, showing that the terminal pH of dual-species inter-kingdom biofilms was below the critical enamel demineralization pH. The pH profiles displayed an exponential decline in the pH of interkingdom dual-species biofilms, dropping from the initial pH of 7 to approximately pH 4.5, two hours into the experiment. This low pH level persisted throughout the experimental timeline.
Figure 4.
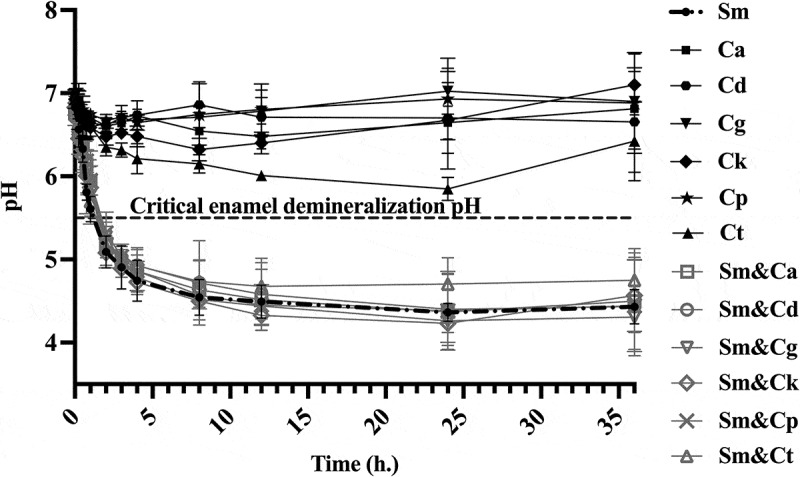
The pH kinetics of mono- and dual-species, inter-kingdom biofilms during 36 hours experimental period. The data are means and standard deviations of three separate experiments (n = 3). The dark-colored lines indicate the pH of mono-species biofilms, whereas the light-colored lines denote the pH of dual-species biofilms. Sm: S. mutans, Ca: C. albicans, Cd: C. dubliniensis, Cg: C. glabrata, Ck: C. krusei, Cp: C. parapsilosis, Ct: C. tropicalis.
In contrast, the pH of all mono-species Candida biofilms remained relatively stable, fluctuating between pH 6 and 7 over the 36-hour experimental period (Figure 4). These results suggest that S. mutans irrespective of the presence of the Candida species maintain its acidogenic potential in interkingdom biofilms.
Evaluation of cariogenic virulence gene expression of Streptococcus mutans in dual-species, interkingdom biofilms
We further investigated the gene expression levels associated with various cariogenic attributes of S. mutans. The expression levels of a total of 13 genes were evaluated which include gtfBCD related to exopolysaccharides (EPS) synthesis, gbpB related to glucan binding protein, genes related to sugar utilization - scrAB, ldh, pflA and pdhA, acid stress tolerance genes - atpD and fabM and finally the ciaRH related to signal transduction system [8,9].
Regarding the genes actively expressed during biofilm formation, the cariogenic gene expression profile of S. mutans in the dual-species biofilms Sm&Ck and Sm&Ct closely resembled that of Sm&Ca, with the exception of Sm&Cd combination where most of the evaluated genes were downregulated (Figure 5).
The gene gtfB which plays a crucial role in S. mutans and C. albicans biofilms were upregulated in all dual-species biofilms except in the case of C. dubliniensis [6]. Similarly, gtfD was upregulated in dual-species biofilms, except in Sm&Cd and Sm&Cp biofilms.
On the other hand, the expression of gtfC remained largely unchanged in most dual-species biofilms, except in the case of Sm&Cd and Sm&Ck biofilms where gene expression was down- and upregulated, respectively. Furthermore, gbpB, involved in sucrose-dependent adherence, was notably downregulated in Sm&Ck, Sm&Cp, and Sm&Ct biofilms.
Sucrose metabolism plays a pivotal role in cariogenicity. Therefore, we scrutinized scrAB gene expression, which encodes the major enzymes IIscr and sucrose-6-phosphate hydrolase, contributing to the phosphoenolpyruvate: sugar phosphotransferase system (PTS) related to sucrose transport and metabolism [26]. This gene exhibited upregulation in all dual-species biofilms except in Sm&Cd and Sm&Cp biofilms (Figure 5).
In environments rich in sugar, pyruvate is typically converted to lactate by lactate dehydrogenase (LDH). Conversely, under conditions of sugar scarcity, pyruvate dehydrogenase (PDH) and pyruvate formate lyase (PFL) are activated, leading to the breakdown of pyruvate into acetyl-CoA, formate, acetate, and/or ethanol [9]. The expression of ldh remained constant across all dual-species biofilms. In contrast, pdhA was upregulated in Sm&Ca, Sm&Ck and Sm&Ct, while being downregulated in Sm&Cd. The expression of pfl showed no significant change in most dual-species biofilms, except for being downregulated in Sm&Cd and unregulated in Sm&Ct (Figure 5).
Furthermore, acid tolerance achieved through proton expulsion and augmentation of monounsaturated fatty acids stands as another crucial cariogenic virulence factor [27]. In this context, the expression of fabM responsible for synthesizing unsaturated membrane fatty acids, remained unaltered in all dual-species biofilms except for Sm&Cd combination, where it was downregulated. In contrast, atpD, which encodes the F-ATPase proton pump, exhibited downregulation in Sm&Cd, Sm&Cg and Sm&Cp with no discernible change observed in the other dual-species biofilms (Figure 5).
Finally, the ciaRH two-component signal transduction system plays a pivotal role in the ecological fitness and cariogenic potency of S. mutans, influencing biofilm formation and acid tolerance [26]. In this regard, only Sm&Ck and Sm&Ct biofilms displayed upregulation of ciaR with its downregulation in Sm&Cd biofilm. In contrast, all dual-species biofilms, except Sm&Cd, exhibited upregulation and expression of ciaH (Figure 5).
Discussion
This study delves into the intricate interplay between Streptococcus mutans and a number of pathogenic NACs within dual-species biofilms. In particular our data, for the first time, shed light on how such interactions could impact the overall biofilm growth and development, pH regulation, and gene expression, in relation to cariogenicity.
A recent meta-analysis of clinical studies have shown a significant association between C. albicans and early childhood caries (ECC) [28]. Compared to caries-free children, those with ECC had higher prevalence of oral colonization with C. albicans, and children with oral Candida had greater odds of developing caries. These clinical observations are consistent with the roles of C. albicans in promoting cariogenesis, which are also supported by several preclinical and laboratory investigations [6–9]. Mechanistically, it has been demonstrated that C. albicans promote biofilm formation and acid production of S. mutans [6–9,29]. Our results are consistent with these findings and provide further insights into the role of the less-studied NACs. However, a few researchers have suggested that C. albicans could also play an anti-cariogenic role in dual-species biofilms of S. mutans, by increasing the pH of the biofilm at late timepoints, particularly at 72 hours of incubation [30]. These imply that the interactions of the dual-species biofilm of Candida species and S. mutans are highly complex and dynamic, and indicate the necessity for further investigations.
The dual-species biofilms of S. mutans (Sm) with C. albicans (Ca), C. glabrata (Cg) and C. tropicalis (Ct) but not the other cohabitant yeasts, were significantly greater than their mono-species counterparts (p < 0.05, Figure 1). It is difficult to offer reasons for this difference as there is limited information available on the interactions of S. mutans with NACs. One reason for this may be because the mannoproteins of each NACs exhibit structural and compositional differences in comparison to that of C. albicans [12–15]. Additionally, the extracellular polymeric substances (EPS) of C. tropicalis are characterized by the presence of hexosamine instead of the typical carbohydrates found in the EPS of C. albicans [31]. These distinctions in mannoprotein structure and EPS composition are likely to play a role in the variations in biofilm mass we observed between different NACs and S. mutans.
With regards to quantitative evaluation of the biofilm cells, we noted a remarkable increase in S. mutans counts when co-cultured with C. albicans (p < 0.0001), whereas there was no significant change in C. albicans numbers. Previous studies quantifying of viable cells in dual-species biofilms of S. mutans and C. albicans have yielded conflicting results. While most reported an increase in the number of S. mutans and C. albicans when co-cultured, some have found no significant difference compared to their respective mono-species biofilms [8,32,33]. Interestingly, we also observed a significant increase in C. tropicalis counts when co-cultured with S. mutans (p < 0.001), despite the absence of significant alterations in S. mutans numbers (Figure 2). Ellepola et al. [6] have proposed a mechanism through which S. mutans likely promotes the proliferation of C. albicans in dual-species biofilms. They surmise that Gtf enzymes of S. mutans, which aid glucan production and also hydrolyse sucrose into glucose and fructose, may offer raw nutrients for candidal growth as the yeasts have a limited capacity to metabolize sucrose.
This metabolic activity also results in acid production, contributing to a decreased pH within the dual-species biofilm, a phenomenon supported by our findings and those of previous workers [32]. In addition, Fakhruddin et al. [10] have recently demonstrated that clinical isolates of C. albicans, C. krusei, C. tropicalis, and C. glabrata exhibit acidogenic capabilities in a glucose-rich ecosystem. Furthermore, Candida species have an innate capacity to produce short-chain carboxylic acids, such as pyruvates and formates, which are likely to help generate acidic biofilms [34]. This exceptional ability of yeasts to produce acids and also grow under low-pH conditions is likely to promote enamel demineralization which is the prime mover in sECC [35].
The foregoing findings were also confirmed in our gene expression studies as we noted the upregulation of most S. mutans genes related to acidogenicity in the interkingdom biofilm, particularly in the case of C. tropicalis- S. mutans combination. However, those related to acid tolerance, i.e. atpD and fabM, were either unchanged or downregulated, mirroring observations seen in C. albicans (Figure 5). Indeed Falsetta et al. [8] have noted that the latter two genes are upregulated only in biofilms aged 48 hours, with reduced expression noted at earlier time points. This may be the reason why we did not detect upregulation of acid tolerance genes as our study lasted only 36 hours.
The mechanisms by which C. albicans augment dual-species biofilm are known to be mediated by the release of the quorum sensing chemical farnesol, which activates S. mutans growth and enhances gtf expression and activity, that in turn synergises glucan production – a key component of the biofilm extracellular polysaccharide [36]. Additionally, C. albicans provides a larger binding area for GtfB and is known to produce β-1,3-glucans, which is likely to enhance the dual-species biofilm formation [8,29]. Concordant with the previous literature [8,37], the co-culture of S. mutans with C. albicans was found to boost biofilm mass and upregulate gtfB expression, with a similar trend observed with C. tropicalis where we noted a substantive increased biofilm mass and gtfBD expression. (Figures 1 and 5).
C. krusei exhibited increased gene expression, particularly gtfC, although this did not correlate with a significantly increased biofilm mass. Perhaps this anomaly could be attributed to its poor biofilm-forming ability and detachment issues on polystyrene plastic surfaces [38].
We also observed that in the presence of C. albicans, the expression of ldh, typically induced under sucrose excess, was unchanged, while pdhA, induced under sucrose limitation, was upregulated. This pattern was similar to that seen in interkingdom biofilms of S. mutans with either C. tropicalis or C. krusei (Figure 5). He et al. [9] have hypothesized that the metabolism of sugars by Candida species could lead to localized sugar limitations, potentially diversifying bacterial carbohydrate metabolism. Furthermore, we observed that Candida species upregulated ciaRH, expression in S. mutans, which controls its cariogenic virulence traits such as biofilm formation and acid tolerance, aligning consistently with previous studies [9]. This finding underscores the significance of the ciaRH system in orchestrating S. mutans’ responses within interkingdom communities. The differential regulation of ciaR and ciaH in various dual-species biofilms suggests intricate variations in signalling pathways and regulation of gene expression that underpin the ecological dynamics and cariogenic potential of S. mutans in polymicrobial environments. These gene expression profiles described above offer valuable insights into the complex interplay between S. mutans and its coexisting species and the multifaceted pathways involved in biofilm formation and cariogenic potential. Nevertheless, further research is warranted to unravel these intriguing interactions within inter-kingdom biofilms.
A previous study found that the expression of the alternative sigma factor that regulates quorum sensing, sigX (also known as comX), was upregulated in S. mutans when co-cultured with C. albicans [39]. In this study we did not examine sigX directly because we focused on genes identified through prior RNA-seq analysis, particularly genes related to carbohydrate transport, sugar metabolism, and two-component systems (TCS) associated with the bacterium’s cariogenic potential. In addition, the upregulation of sigX in dual-species biofilm was highly dynamic, with the peak of expression at 10 hours of biofilm incubation and was downregulated by 24 hours. Thus, the expression of sigX may not be apparent in our biofilm growth conditions of 36 hours. Nevertheless, we observed that ciaH was upregulated, particularly in the co-culture of S. mutans and C. tropicalis. The latter gene regulates the expression of comC, which subsequently controls the comED system; activation of comE can, in turn, enhance the expression of sigX [40,41]. This is consistent with an activation of the quorum sensing pathway.
Several studies have found C. dubliniensis to be more abundant in children with early childhood caries (ECC) compared to caries-free individuals, with its levels increasing as caries progress [42,43]. However, its specific role in the development of dental caries remains unknown. Interestingly, our study revealed that the presence of C. dubliniensis led to the downregulation of key cariogenic genes in S. mutans (Figure 5). This unexpected finding highlights the need for further investigation into the role of C. dubliniensis in dental caries and its potential impact on bacterial pathogenicity.
Finally, we recognize the constraints of our biofilm model in replicating the intricate structure of the natural tooth surface and the oral ecosystem. Given that our model was designed to delve into microbial interactions and gene expression within a controlled environment our results offer fundamental insights for further investigations.
To conclude, our study underscores the cariogenic potential of the interkingdom biofilm of S. mutans and NACs, particularly those of C. tropicalis. Our in vitro observations, in general, resonate with the clinical findings of Fakhruddin et al. [10] which clearly show the predominance of C. tropicalis, surpassing even the more virulent C. albicans, in deep dentinal lesions of sECC. Recognising the mechanistic interactions between such interkingdom biofilms between S. mutans and Candida species is critical for future studies aimed at improving strategies for caries prevention and management. Moreover, our study characterizes the intricate dynamics of dual-species, interkingdom biofilms, shedding light on how the behaviour of the microbial constituents is influenced by their neighbourly cohabitants, driving microbial proliferation and metabolic activities within the biofilm matrix.
Conclusions
This study provides evidence for the first time that among NACs, Candida tropicalis, in particular, demonstrates the highest cariogenic potential when coexisting in a dual-species biofilms with Streptococcus mutans. This finding is supported by the gene expression profile of S. mutans and the observed phenotypes of the biofilms, such as increased biofilm mass and low pH levels associated with enamel demineralization. Further exploration of the inter-kingdom interactions between Candida and Streptococcus mutans is crucial to developing effective strategies for the prevention and management of dental caries.
Acknowledgments
I acknowledge using ChatGPT version 1.2024.346 to refine the writing style.
Funding Statement
This work was supported by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund) and Ratchadaphiseksomphot Endowment Fund to Center of Excellence on Oral Microbiology and Immunology, Chulalongkorn University. Professor Lakshman Samaranayake was supported by the Chulalongkorn University, second century (C2) high potential professoriate fund at its Faculty of Dentistry.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions statement
W.S. participated in conducting experiments, data analysis and interpretation, and drafted the initial manuscript; O.M. participated in study design, supervised data analysis, data interpretation and critically revised the manuscript; M.S. participated in conducting experiments; L.S. participated in data interpretation and critically revised the manuscript; P.T. participated in study design, supervised data collection and analysis, interpreted the data, drafted and critically revised the manuscript. All the authors have read and approved the final manuscript.
Data availability statement
The data generated in the current study are available from the corresponding author on reasonable request.
References
- [1].Kahharova D, Pappalardo VY, Buijs MJ, et al. Microbial indicators of dental health, dysbiosis, and early childhood caries. J Dent Res. 2023;102(7):759–11. doi: 10.1177/00220345231160756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pereira D, Seneviratne CJ, Koga-Ito CY, et al. Is the oral fungal pathogen Candida albicans a cariogen? Oral Dis. 2018;24(4):518–526. doi: 10.1111/odi.12691 [DOI] [PubMed] [Google Scholar]
- [3].Fakhruddin KS, Perera Samaranayake L, Egusa H, et al. Candida biome of severe early childhood caries (S-ECC) and its cariogenic virulence traits. J Oral Microbiol. 2020;12(1):1724484. doi: 10.1080/20002297.2020.1724484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Banas JA. Virulence properties of Streptococcus mutans. Front Biosci. 2004;9(1–3):1267–1277. doi: 10.2741/1305 [DOI] [PubMed] [Google Scholar]
- [5].Xiang Z, Wakade RS, Ribeiro AA, et al. Human tooth as a fungal niche: Candida albicans traits in dental plaque isolates. MBio. 2023;14(1):e0276922. doi: 10.1128/mbio.02769-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ellepola K, Truong T, Liu Y, et al. Multi-omics analyses reveal synergistic carbohydrate metabolism in streptococcus mutans-Candida albicans mixed-species biofilms. Infect Immun. 2019;87(10). doi: 10.1128/IAI.00339-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hwang G, Liu Y, Kim D, et al. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLOS Pathog. 2017;13(6):e1006407. doi: 10.1371/journal.ppat.1006407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Falsetta ML, Klein MI, Colonne PM, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82(5):1968–1981. doi: 10.1128/IAI.00087-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].He J, Kim D, Zhou X, et al. RNA-Seq reveals enhanced sugar metabolism in Streptococcus mutans Co-cultured with Candida albicans within mixed-species biofilms. Front Microbiol. 2017;8:1036. doi: 10.3389/fmicb.2017.01036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fakhruddin KS, Perera Samaranayake L, Egusa H, et al. Profuse diversity and acidogenicity of the candida-biome of deep carious lesions of severe early childhood caries (S-ECC). J Oral Microbiol. 2021;13(1):1964277. doi: 10.1080/20002297.2021.1964277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].de Carvalho FG, Silva DS, Hebling J, et al. Presence of mutans streptococci and Candida spp. In dental plaque/dentine of carious teeth and early childhood caries. Arch Oral Biol. 2006;51(11):1024–1028. doi: 10.1016/j.archoralbio.2006.06.001 [DOI] [PubMed] [Google Scholar]
- [12].Kobayashi H, Matsuda K, Ikeda T, et al. Structures of cell wall mannans of pathogenic Candida tropicalis IFO 0199 and IFO 1647 yeast strains. Infect Immun. 1994;62(2):615–622. doi: 10.1128/iai.62.2.615-622.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takahashi S, Kudoh A, Okawa Y, et al. Significant differences in the cell-wall mannans from three Candida glabrata strains correlate with antifungal drug sensitivity. FEBS J. 2012;279(10):1844–1856. doi: 10.1111/j.1742-4658.2012.08564.x [DOI] [PubMed] [Google Scholar]
- [14].Shibata N, Ikuta K, Imai T, et al. Existence of branched side chains in the cell wall mannan of pathogenic yeast, Candida albicans. Structure-antigenicity relationship between the cell wall mannans of Candida albicans and Candida parapsilosis. J Biol Chem. 1995;270(3):1113–1122. doi: 10.1074/jbc.270.3.1113 [DOI] [PubMed] [Google Scholar]
- [15].Shibata N, Kobayashi H, Suzuki S.. Immunochemistry of pathogenic yeast, Candida species, focusing on mannan. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(6):250–265. doi: 10.2183/pjab.88.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Navarro-Arias MJ, Hernandez-Chavez MJ, Garcia-Carnero LC, et al. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect Drug Resist. 2019;12:783–794. doi: 10.2147/IDR.S197531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arzmi MH, Alnuaimi AD, Dashper S, et al. Polymicrobial biofilm formation by Candida albicans, Actinomyces naeslundii, and Streptococcus mutans is Candida albicans strain and medium dependent. Med Mycol. 2016;54(8):856–864. doi: 10.1093/mmy/myw042 [DOI] [PubMed] [Google Scholar]
- [18].Barbosa JO, Rossoni RD, Vilela SF, et al. Streptococcus mutans can modulate biofilm formation and attenuate the virulence of Candida albicans. PLOS ONE. 2016;11(3):e0150457. doi: 10.1371/journal.pone.0150457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cury JA, Koo H. Extraction and purification of total RNA from Streptococcus mutans biofilms. Anal Biochem. 2007;365(2):208–214. doi: 10.1016/j.ab.2007.03.021 [DOI] [PubMed] [Google Scholar]
- [20].Wang Y, Wang X, Jiang W, et al. Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. J Oral Microbiol. 2018;10(1):1442089. doi: 10.1080/20002297.2018.1442089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wen ZT, Yates D, Ahn S-J, et al. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010;10(1):111. doi: 10.1186/1471-2180-10-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li B, Li X, Lin H, et al. Curcumin as a promising antibacterial agent: effects on metabolism and biofilm formation in S. mutans. Biomed Res Int. 2018;2018:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bezerra DS, Stipp RN, Neves BG, et al. Insights into the virulence traits of Streptococcus mutans in dentine carious lesions of children with early childhood caries. Caries Res. 2016;50(3):279–287. doi: 10.1159/000445256 [DOI] [PubMed] [Google Scholar]
- [24].Hu X, Wang Y, Gao L, et al. The impairment of methyl metabolism from luxS mutation of Streptococcus mutans. Front Microbiol. 2018;9:404. doi: 10.3389/fmicb.2018.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Park SN, Lim YK, Kook JK. Development of quantitative real-time PCR primers for detecting 42 oral bacterial species. Arch Microbiol. 2013;195(7):473–482. doi: 10.1007/s00203-013-0896-4 [DOI] [PubMed] [Google Scholar]
- [26].He LY, Le YJ, Guo Z, et al. The role and regulatory network of the CiaRH two-component system in Streptococcal species. Front Microbiol. 2021;12:693858. doi: 10.3389/fmicb.2021.693858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fozo EM, Quivey RG Jr.. The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH. J Bacteriol. 2004;186(13):4152–4158. doi: 10.1128/JB.186.13.4152-4158.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xiao J, Huang X, Alkhers N, et al. Candida albicans and early childhood caries: a systematic review and meta-analysis. Caries Res. 2018;52(1–2):102–112. doi: 10.1159/000481833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Khoury ZH, Vila T, Puthran TR, et al. The role of Candida albicans secreted polysaccharides in augmenting Streptococcus mutans adherence and mixed biofilm formation: in vitro and in vivo studies. Front Microbiol. 2020;11:307. doi: 10.3389/fmicb.2020.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Willems HM, Kos K, Jabra-Rizk MA, et al. Candida albicans in oral biofilms could prevent caries. Pathog Dis. 2016;74(5):ftw039. doi: 10.1093/femspd/ftw039 [DOI] [PubMed] [Google Scholar]
- [31].Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55(8):999–1008. doi: 10.1099/jmm.0.46569-0 [DOI] [PubMed] [Google Scholar]
- [32].Lobo CIV, Rinaldi TB, Christiano CMS, et al. Dual-species biofilms of Streptococcus mutans and Candida albicans exhibit more biomass and are mutually beneficial compared with single-species biofilms. J Oral Microbiol. 2019;11(1):1581520. doi: 10.1080/20002297.2019.1581520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Eidt G, Andrade CG, Negrini TC, et al. Role of Candida albicans on enamel demineralization and on acidogenic potential of Streptococcus mutans in vitro biofilms. J Appl Oral Sci. 2019;27:e20180593. doi: 10.1590/1678-7757-2018-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Samaranayake LP, Hughes A, Weetman DA, et al. Growth and acid production of Candida species in human saliva supplemented with glucose. J Oral Pathol. 1986;15(5):251–254. doi: 10.1111/j.1600-0714.1986.tb00617.x [DOI] [PubMed] [Google Scholar]
- [35].Klinke T, Kneist S, de Soet JJ, et al. Acid production by oral strains of Candida albicans and lactobacilli. Caries Res. 2009;43(2):83–91. doi: 10.1159/000204911 [DOI] [PubMed] [Google Scholar]
- [36].Kim D, Sengupta A, Niepa TH, et al. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep. 2017;7(1):41332. doi: 10.1038/srep41332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xiao J, Zeng Y, Rustchenko E, et al. Dual transcriptome of Streptococcus mutans and Candida albicans interplay in biofilms. J Oral Microbiol. 2023;15(1):2144047. doi: 10.1080/20002297.2022.2144047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Diaz-Garcia J, Marcos-Zambrano LJ, Munoz P, et al. Does the composition of polystyrene trays affect Candida spp. biofilm formation? Med Mycol. 2019;57(4):504–509. doi: 10.1093/mmy/myy064 [DOI] [PubMed] [Google Scholar]
- [39].Sztajer H, Szafranski SP, Tomasch J, et al. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J. 2014;8(11):2256–2271. doi: 10.1038/ismej.2014.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ahn SJ, Wen ZT, Burne RA. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect Immun. 2006;74(3):1631–1642. doi: 10.1128/IAI.74.3.1631-1642.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].van der Ploeg JR. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J Bacteriol. 2005;187(12):3980–3989. doi: 10.1128/JB.187.12.3980-3989.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Al-Ahmad A, Auschill TM, Dakhel R, et al. Prevalence of Candida albicans and Candida dubliniensis in caries-free and caries-active children in relation to the oral microbiota—a clinical study. Clin Oral Investig. 2016;20(8):1963–1971. doi: 10.1007/s00784-015-1696-9 [DOI] [PubMed] [Google Scholar]
- [43].O’Connell LM, Santos R, Springer G, et al. Site-specific profiling of the dental mycobiome reveals strong taxonomic shifts during progression of early-childhood caries. Appl Environ Microbiol. 2020;86(7). doi: 10.1128/AEM.02825-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the current study are available from the corresponding author on reasonable request.


