Abstract
The aim of this study was to compare the phenolic compound content and antioxidant activity of infusions made from commercially available Morus alba L. leaves with those of infusions made from a naturally collected source. The phenolic profile was determined using RP-HPLC–DAD and LC-Q-TOF–MS/MS. The total phenolic content (TPC) was determined using Folin-Ciocâlteu reagent. To assess the antioxidant and reducing properties, Trolox equivalent antioxidant capacity (TEAC) and ferric reducing antioxidant power (FRAP) were used. Our analysis revealed the presence of two phenolic acids and six flavonoids. The most abundant compound was chlorogenic acid. The TPC, TEAC, and FRAP results indicated that infusions prepared from naturally collected samples exhibited greater phenolic content (19.7—52.6 vs 18.1—35.2 mg/100 ml) and stronger antioxidant (0.0605—0.1842 vs 0.0453—0.0822 mmol Trolox/100 ml) and reducing (0.244—0.597 vs 0.202—0.371 mmol Fe2+/100 ml) activities than those of commercially available products in the Polish market. Samples L1-L3 from the natural collection were those with the highest values. These results were further supported by principal component analysis (PCA), which categorized observations into three distinct clusters.
Keywords: Morus alba L., Phenolic compounds, Antioxidant activity, RP-HPLC-DAD, Chlorogenic acid, LC-Q-TOF-MS/MS
Subject terms: Secondary metabolism, Disease prevention, Lifestyle modification, Nutritional supplements, Preventive medicine, Nutrition, Therapeutics, Nutrition therapy, Diabetes complications, Type 2 diabetes
Introduction
Morus alba L., commonly known as white mulberry, is a deciduous woody tree belonging to the Moraceae family that was originally harvested from Asia. Its leaves have been integral to Traditional Chinese Medicine for over three millennia and are utilized to address various ailments, including diabetes, cancer, and cold1. They are commercially available as tea or beverages in many countries, while in certain regions of China, young leaves are consumed as vegetables. The adaptability of white mulberry to diverse climatic and soil conditions, its resilience to air pollutants, and its resistance to diseases and pests have led to its introduction into various temperature zones in Europe, North and South America, and Africa2.
In recent years, there has been an escalating demand for plant-derived constituents and products, prized for their applications in functional foods, nutraceuticals, drug development, and discovery3–7. Consequently, materials such as white mulberry leaves have garnered significant interest due to their status as valuable nutrient sources for extraction and incorporation into food formulations8–11.
The bioactive compounds present in white mulberry leaves are known for their potential as effective α-glucosidase inhibitors with the capacity to induce hypoglycaemic effects12,13. The literature also suggests the feasibility of using white mulberry leaf extracts to address other conditions, such as hypercholesterolemia14. Due to these properties, functional tea crafted from white mulberry leaves has gained popularity as a beverage15.
Moreover, the bioactive molecules found within white mulberry leaves may play a crucial role in mitigating myocardial infarction and hypertension through the regulation of antioxidant defensive mechanisms16. In diabetic animals, the consumption of mulberry leaf extract significantly enhanced the activities of enzymes involved in the antioxidative defence system13. Both mulberry leaf extracts and isolated compounds exhibit cytotoxicity against various human cancer cell lines, including HeLa cervical carcinoma cells, MCF- 7 breast carcinoma cells, Hep3B hepatocarcinoma cells, and HepG2 hepatoma cells17,18. Previous reports have discussed the antiobesity, anti-inflammatory, and antiatherosclerotic potential of mulberry leaves13. These multifaceted properties are attributed to the high concentrations of bioactive compounds, such as phenolic acids, flavonoids, proteins, organic acids, and macronutrients. Notably, these compounds include chlorogenic acid, rutin, isoquercitrin, astragalin, and 1-deoxynojirimycin9,13,19–23.
Due to their valuable nutritional composition and versatile pharmacological properties, mulberry leaves have extensive applications in the food industry, dietary supplements, pharmaceuticals, and cosmetic production.
In Poland, dietary supplements incorporating white mulberry leaves have been very popular for many years mainly among diabetes and prediabetes patients to maintain a normal blood glucose homeostasis. They are easy accessible and affordable nutritional products, available mainly in a wide range of ready-to-drink tea beverages rather than solid forms (capsules and tablets). A regular practice among patients with impaired glucose management system is used concomitantly white mulberry leaves dietary products with conventional hypoglycemic agents to increase the effectiveness of therapy and help to achieve therapeutic goals and reduce adverse effects. According to manufacturers, an infusion of 2.0 g of dried mulberry leaves, consumed 2–3 times daily, can have hypoglycaemic effects. However, there remains a knowledge gap regarding the composition and quantities of the active constituents in mulberry leaf supplements. Additionally, the antioxidant potential of mulberry teas available on the Polish market remain unclear.
Unfortunately, in Poland, the reliable control of each food supplement is a big challenge. There are lack of detailed studies and requirements on food supplements sold in Polish market, confirming the composition in line with the label and safety for the consumer. Moreover, there is also no obligation to test the content of active substances or to standardize the raw material for specific active substances in these products24. Those information are crucial with respect to both safety and efficacy of dietary supplements in the treatment of diabetes. Laboratory tests commissioned by The Supreme Audit Office (state institution in Poland) revealed that many supplements do not possess the qualities declared by the manufacturers, while some of them are even harmful to health. The checks showed the presence of supplements containing i.a pathogenic bacteria or banned substances from the list of psychoactive drugs25. In addition, much lower requirements in the manufacturing, controlling and marketing of dietary supplements compared to synthetic drugs may eventually lead to changes to quality and quantity of ingredients within products. Worth of attention is also complications of combined pharmacotherapy due to possible pharmacokinetic and pharmacodynamic interactions between conventional drugs and plant derived products metabolites.
Therefore, based on the aforementioned considerations, the primary goal of our research was to carry out analysis of the composition and antioxidant potential of food supplements that incorporate dried white mulberry leaves available on the Polish market and compare them with with those derived from natural collections. To the best of our knowledge, there is no clear information in recent literature about quality assessment and antioxidant properties of products sold as dietary supplements with Morus alba leaves in Poland. Additionally, some reports about available products seem to be insufficient in terms of phenolic compounds profile, when compared with reports from other regions26,27 This study aimed to provide crucial insights into the antioxidant potential and phenolic composition of these supplements, with a particular emphasis on elucidating any variations that might exist between commercially available products and those obtained from natural sources. We believe that consumers need and deserve to have access to detailed and necessary product information to help them use products safely, as well as avoid interaction between food-food supplements, drugs-food supplements, gene-food supplements, and minimize the risk of their harmful and allergic consequences. In our opinion, in the case of herbal substances or herbal preparations with constituents of known therapeutic activity, clear and accurate product labelling including identification and quantitation of active substances, as well as identification of unauthorized compounds empowers consumers to make informed, wise and first of all health-promoting choices. Moreover, by addressing this knowledge gap, our research sought to contribute valuable data that could not only inform both consumers and the industry about the nutritional and pharmacological attributes of mulberry leaf-based supplements, provide suggestions on the consumption and regulations of supplements in order to reduce the adverse effect cases caused by them and facilitating more informed choices and product development strategies as well further research with mulberry-based products.
Materials and methods
Chemicals
All reagents used in the study were of the highest analytical grade, and the standard solutions were prepared with deionized water. Quercetin, quercetin- 3-O-rutinoside (rutin), quercetin- 3-O-glucoside (isoquercitrin), kaempferol- 3-O-glucoside (astragalin), chlorogenic acid, caffeic acid, Folin–Ciocâlteu’s phenol reagent, formic acid, sodium persulfate, phosphate-buffered saline, 2,2’-azinobis(3-ethylbenzothiazoline- 6-sulfonic acid) (ABTS), 6-hydroxy- 2,5,7,8-tetramethyl-chroman- 2-carboxylic acid (Trolox), (+)-catechin, sodium carbonate, ferrous chloride, acetate buffer, and 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) were obtained from Sigma Aldrich (St. Louis, MO, USA). Acetonitrile, methanol and trifluoroacetic acid were acquired from Avantor Performance Materials (Gliwice, Poland). Water was purchased from Merck (Darmstadt, Germany).
Plant material and preparation of samples
The research material consisted of 15 herbal medicines containing white mulberry leaves, as shown in Table 1. Among them, 9 were purchased locally in pharmacies and herbal stores in Kuyavian-Pomeranian Voivodeship (M1-M9). They were stored in their original packaging until analysis and are reffered as ‘comercial products’. The other 3 herbage samples of mulberry leaves, L1-L3, were harvested from trees located near the complex of Antoni Jurasz University Hospital No. 1 in Bydgoszcz (Kuyavian-Pomeranian Voivodeship, Poland). Plant verification and identification were performed by Assoc. Prof. Anna Kurzyńska-Kokorniak, PhD, DSc (Department of Ribonucleoprotein Biochemistry, Institute of Bioorganic Chemistry Polish Academy of Sciences Poznań, Poland) using the intersimple sequence repeat polymerase chain reaction technique (ISSR-PCR). The leaves were collected at the end of May in the afternoon (1–2 p.m.) on sunny days, preceded by a rainless period lasting at least a few days. The harvested mulberry leaves were gently cleaned to remove dirty materials and immediately dried under natural conditions (on plain paper in a ventilated place without direct sunlight). The dried samples were transferred to paper bags and kept at room temperature in the dark until use to prevent any physical or microbial damage. The remaining 3 products (L4-L6) were donated by a Polish pharmaceutical company (Natur-Vit, Pińczów, Poland). Samples were collected from Pakistan, China and Poland. Samples L1-L6 are referred as ‘raw plant material’. All the samples (n = 15) were grouped into two collections: commercial products (M1-M9, n = 9) and raw plant material (L1-L6, n = 6).
Table 1.
The characteristics of the 15 analysed samples containing white mulberry leaves.
| Sample | Origin | Form |
|---|---|---|
| Commercial products | ||
| M1 | Poland, China | Tea bags |
| M2 | Poland | Tea bags |
| M3 | Poland | Tea bags |
| M4 | Poland | Tea bags |
| M5 | Bulgaria | Tea bags |
| M6 | Poland | Tea bags |
| M7 | Bulgaria | Tea bags |
| M8 | China | Tea bags |
| M9 | Turkey, Bulgaria | Tea bags |
| Raw plant material | ||
| L1 | Poland | Loose leaf |
| L2 | Poland | Loose leaf |
| L3 | Poland | Loose leaf |
| L4 | China | Loose leaf |
| L5 | Pakistan | Loose leaf |
| L6 | Poland | Loose leaf |
Extraction
Two grams of leaves were infused with 100 mL of freshly boiled deionized water for 5 min. Next, the extract was decanted, filtered through Chromafil PES 45/25 single-use syringe filters (0.45 µm, Macherey–Nagel, Düren, Germany) and adjusted to ambient temperature.
Identification and quantification of the phenolic compounds of Morus alba
Liquid chromatography quadrupole time-of-flight tandem mass spectrometry (LC-Q-TOF–MS/MS)
Phenolic compounds were identified28 using an Eksigent microLC 200 system coupled with a TripleTOF 5600 + mass spectrometer (AB Sciex, Framingham, MA, USA). Chromatographic separation was carried out on an Eksigent Halo C18 column (0.5 × 50 mm, 2.7 µm; AB Sciex). The mobile phase consisted of 0.1% (v/v) formic acid in water (solvent A) and 0.1% (v/v) formic acid in acetonitrile (solvent B), using a linear gradient system that increased the proportion of solvent B from 1 to 90% over 3 min. The electrospray ionization source (ESI) was operated in negative ion mode, with an ion spray voltage of 4.5 kV. The nebulizer gas (GS1) and curtain gas flow rates were set to 30 L/min, while the heater gas (GS2) flow rate was 35 L/min. The turbo spray temperature was maintained at 350 °C. For full-scan MS, the declustering potential (DP) was set to − 90 or 90 V, and the collision energy (CE) was − 20 or 20 eV. For MS2 mode, the DP and CE values were adjusted to − 80 or 80 V and − 30, − 10 or 10 eV, respectively. The time-of-flight (TOF) mass spectrometer was operated in the 100–1200 m/z mass range.
Reversed phase high performance liquid chromatography with diode-array detector (RP-HPLC–DAD) analysis
The separation of phenolic compounds11,29 was carried out by using the RP-HPLC–DAD Shimadzu Nexera X2 system (Shimadzu, Kyoto, Japan), which was equipped with a CBM- 20 A system controller, a CTO- 20 AC column oven, a DGU- 20 A5R degassing unit, two LC- 30 AD pumps, a SIL- 30 AC autosampler, and an SPD-M30 A photodiode array detector. Phytochemical separation was accomplished using a Luna Omega C18(2) column (4.6 × 150 mm, 3 µm; Phenomenex, Torrance, CA, USA). The analyte was separated at 25 °C in binary gradient mode using the following mobile phases to determine the phenolic content: solvent A, acetonitrile–water-trifluoroacetic acid (5:95:0.1; v/v/v); solvent B, acetonitrile-trifluoroacetic acid (100:0.1; v/v) for 0–10 min, 0–25% B; 10–15 min, 60% B; 15–17 min, 0% B; and 17–21 min, 0% B. The flow rate was 1 mL/min. The samples were filtered (0.22 µm, polyethersulfone membrane filter, TPP Techno Plastic Products AG, Trasadingen, Switzerland) and injected (10 µl). The detection of phenolics by UV–Vis spectra means of the photodiode detector was monitored over a wavelength range of 200–400 nm. Phenolic quantification by the external standard method was carried out using caffeic acid, chlorogenic acid, quercetin- 3-O-rutinoside (rutin), quercetin- 3-O-glucoside (isoquercitrin), kaempferol- 3-O-glucoside (astragalin), kaempferol and quercetin at a wavelength of 360 nm. The content of the studied compound was expressed as mg/100 ml of infusion (mg/100 mL). All measurements were performed in triplicate.
Determination of total phenolic content (TPC)
A colorimetric method with Folin-Ciocâlteu’s reagent (FCR) was used to determine the total phenolic content30. First, 0.25 ml of sample, 0.25 ml of FCR, 0.5 ml of saturated sodium carbonate solution and 4 ml of water were mixed. The reaction mixture was centrifuged (5 min, 5000 × g, MPW- 350R, MPW Med. Instruments, Warsaw, Poland) after 25 min of the reacting time. Supernatants were subjected under absorbance measurement at 750 nm (DU- 7500 spectrophotometer, Beckman Instruments, Fullerton, CA, USA). The concentrations of TPC were expressed as mg of catechin equivalent (CAE) per 100 ml of infusion (mg CAE/100 ml). Measurements were performed in triplicate.
Determination of 2,2’-azinobis(3-ethylbenzothiazoline- 6-sulfonic acid) (ABTS) radical cation scavenging capacity—Trolox equivalent antioxidant capacity (TEAC)
The ability of white mulberry infusions to quench the synthetic radical cation ABTS was measured according to the procedure proposed by Re et al.31. Following original protocol, ABTS• + was generated and diluted. 2 ml of ABTS• +, 20 µl of sample were mixed and incubated (TH- 24 block heater, Meditherm, Warsaw, Poland) in 30 °C for 6 min. Absorbance was measured at 734 nm. The results are expressed as Trolox equivalents and are presented as mmol Trolox per 100 mL of infusion (mmol Trolox/100 mL).
Determination of ferric reducing antioxidant power (FRAP)
A method described previously by Benzie and Strain32 was used to measure the ability of white mulberry infusions to reduce iron Fe3+ to Fe2+. FRAP reagent was prepared before the analysis. Next, 2.25 ml of the reagent was mixed with 225 µl of water and 75 µl of sample. Reacting mixture was incubated in 37 °C for 30 min and after absorbance was measured at 593 nm. The results are expressed as mmol Fe2+/100 ml of infusion (mmol Fe2+/100 ml).
Statistical analysis
Analysis of the results was performed using statistical inference methods33–35. The basic statistical descriptors included the mean value x and standard deviation (± SD). The normality of the distribution was tested with the Kolmogorov–Smirnov test, while the equality of variance in different samples was tested with the Levene test. Analysis of variance was used to find significant differences between the means, and in the case of significant differences, Tukey’s post hoc test was employed. Correlations among the samples were evaluated using Pearson’s correlation analysis and principal component analysis36. The level of significance for all the statistical tests was set at α = 0.05. The statistical calculations mentioned above were carried out with MS Excel 2019 software (Microsoft, Redmond, WA, USA, 2019) and Statistica 13.3 (Dell, Round Rock, TX, USA, 2022) and PAST 3.2 (Hammer UiO, Norway, 2018) software.
Results and discussion
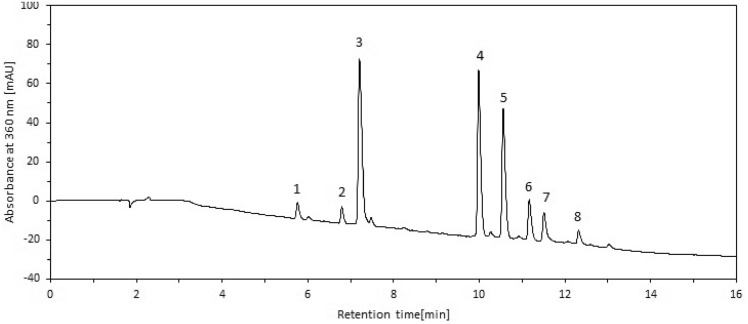
The analysis of individual phenolic compounds in Morus alba (white mulberry) leaf extract was conducted using RP-HPLC–DAD and LC-Q-TOF–MS/MS. A total of eight compounds were identified. Typical chromatogram of the extract of Morus alba is presented in the Fig. 1. Their UV–Vis absorbance maxima (λmax), molecular ions, and fragment ions used for characterization are presented in Table 2. For the identification of phenolic acids we used negative ionisation mode and for flavonoids both negative as well as positive ionisation modes in case of LC-Q-TOF–MS/MS.
Fig. 1.
Typical chromatogram of the extract of Morus alba using RP-HPLC–DAD. 1–3-caffeoylquinic acid (neochlorogenic acid); 2 – quercetin- 3,7-O-di-hexoside; 3–5-caffeoylquinic acid (chlorogenic acid); 4 – quercetin- 3-O-rutinoside (rutin); 5 – quercetin- 3-O-glucoside (isoquercitrin); 6 – quercetin- 3-O-(6’’-O-malonyl)-glucoside; 7 – kaempferol- 3-O-glucoside (astragalin); 8 – kaempferol 3-O-(6’’-O-malonyl)-glucoside.
Table 2.
UV–Vis absorption maxima (λmax) and fragmentation patterns of phenolic compounds identified in Morus alba leafs extracts.
| Peak | λmax (nm) | Collision energy (eV) | Molecular ion (m/z) | Fragment ions (m/z) | Compound |
|---|---|---|---|---|---|
| 1 | 325 | − 10 | 353 | 191, 179, 173, 135 | 3-Caffeoylquinic acid (neochlorogenic acid) |
| 2 | 257, 359 | − 10 | 625 | 463, 301 | Quercetin- 3,7-O-di-hexoside |
| 3 | 325 | − 10 | 353 | 191, 179 | 5-Caffeoylquinic acid (chlorogenic acid) |
| 4 | 257, 356 | − 10 | 609 | 301 | Quercetin- 3-O-rutinoside (rutin) |
| 10 | 611 | 303 | |||
| 5 | 257, 354 | − 10 | 463 | 301 | Quercetin- 3-O-glucoside (isoquercitrin) |
| 10 | 465 | 303 | |||
| 6 | 257, 355 | − 30 | 549 | 505, 300 | Quercetin- 3-O-(6’’-O-malonyl)-glucoside |
| 10 | 551 | 303 | |||
| 7 | 265, 348 | − 30 | 447 | 327, 284 | Kaempferol- 3-O-glucoside (astragalin) |
| 10 | 449 | 287 | |||
| 8 | 268, 348 | − 30 | 533 | 489, 285 | Kaempferol 3-O-(6’’-O-malonyl)-glucoside |
| 10 | 535 | 287 |
Peaks 1, 2, 6, and 8 were tentatively identified. Compounds 3, 4, 5, and 7 were identified using reference standards during RP-HPLC–DAD analysis. We observed two caffeoylquinic acid derivatives: 3-caffeoylquinic acid (neochlorogenic acid) (peak 1) and 5-caffeoylquinic acid (chlorogenic acid) (peak 3) characterized by their fragment ions at m/z 191 and 179. The presence of a fragment ion at m/z 135 in the spectrum of peak 1 suggests that it corresponds to neochlorogenic acid. In contrast, the absence of this fragment in peak 3 supports its identification as chlorogenic acid28,37. Six compounds attributed to flavonoids were identified, including four quercetin derivatives (peaks 2, 4–6) and two kaempferol derivatives (peaks 7 and 8). Observed UV spectra, with λmax at 257 and 354–359 nm for quercetin derivatives and 265–268 and 348 nm for kaempferol derivatives, are characteristic of these two flavonoid classes. The quercetin derivatives exhibited fragment ions at m/z 300, 301, or 303, corresponding to the quercetin aglycone, while kaempferol derivatives showed diagnostic fragment ions at m/z 284, 285, or 287. Among the glycosylated flavonoids, quercetin- 3-O-rutinoside (rutin), quercetin- 3-O-glucoside (isoquercitrin, peak 5), and kaempferol- 3-O-glucoside (astragalin) fragmentation resulted in ions characteristic of these compounds, derived from the loss of sugar moieties. For rutin, the loss of rhamnose-glucose (− 308 Da) was observed, whereas isoquercitrin and astragalin exhibited a loss of glucose (− 162 Da). Peak 2, with a signal at m/z 625, was identified as quercetin- 3,7-O-di-hexoside. This identification is based on the progressive cleavage of glycosidic bonds between the quercetin core and the two sugar moieties, with each cleavage resulting in characteristic fragment ions at m/z 463 (− 162 Da) and 301 (− 162 Da and − 162 Da). Peaks 6 and 8 were attributed to quercetin- 3-O-(6’’-O-malonyl)-glucoside and kaempferol- 3-O-(6’’-O-malonyl)-glucoside, respectively. These compounds displayed fragments resulting from the decarboxylation of the malonyl group (− 44 Da) and the loss of malonyl-glucose (− 284 Da). These findings align with previous research conducted by other authors, including Jeong et al.8, Sánchez-Salcedo et al.21,38, Hu et al.22, Dugo et al.26, Ju et al.39, Li et al.40, Pothinuch et al.41 providing a substantial basis for the thorough examination of phenolic compounds within white mulberry infusion.
The chromatographic separation of phenolic compounds from mulberry leaves is exemplified by typical chromatograms, as presented in studies conducted by Sánchez-Salcedo et al.21, Dugo et al.26, Polumackanycz et al.9,27, Ju et al.39 and Pothinuch et al.41. The outcomes reported by the21,26,39, closely resemble our own findings, with a visual inspection revealing comparable elution patterns. Notably, the primary focus of39was the analysis of flavonoids; however, an unassigned, distinct peak with a relatively short retention time is discernible at the outset of the chromatogram. In our interpretation, this peak likely corresponds to a phenolic acid compound, which elutes earlier than the monoglycosides of flavonoids, and in our study, this compound was identified as chlorogenic acid, distinguished by a strong signal in the chromatogram. Similar findings are presented in21and41. Conversely, the second research group, as delineated by Polumackanycz et al.9,27, presented chromatograms for both hydromethanolic and aqueous extracts that exhibited significant differences from our findings. Latter study was based on samples of Morus alba obtained from Polish market. In both cases, the dominant compounds were phenolic acids. Rutin and aglycones were the only flavonoids identified in those samples. These variations are attributed to differences in their extraction methodologies. In the hydromethanolic extraction performed by the abovementioned team, a solid‒liquid ratio of 1:4 was employed, while for the aqueous extraction, a ratio of 1:200 was utilized. In contrast39, opted for a 1:10 ratio for hydromethanolic extraction, whereas our study utilized a ratio of 1:50, which closely resembles the preparation of a home infusion. Differences between the methodologies of the abovementioned teams manifest in noticeable differences in the obtained outcomes. Moreover, it is worth noting that Polumackanycz et al.9,27 did not identify any glycosides apart from rutin. However, aglycones apigenin and quercetin were identified in Morus. alba leaves from the Polish market27, along with myricetin and naringenin in Morus. alba leaves collected in Italy9. Ju et al.39 suggested that not all flavonoids, particularly acylated compounds, may be present in all Morus alba varieties. In our study, we observed two peaks that were identified as acylated compounds.
Chan et al.42 comprehensively summarized the phenolic profile of Morus alba in their review, which included naringenin but did not mention apigenin or myricetin. Nevertheless, they listed 6-geranylapigenin and 8-geranylapigenin, suggesting that mulberry may have the capacity to biosynthesize these molecules. This inference is supported by the work of Hu et al.22, whose research team provided compelling evidence for the accumulation of apigenin and naringenin glycosides in white mulberry leaves.
However, in our study, the absence of those compounds could be attributed to the use of an aqueous extraction method and, potentially, their low concentrations. Notably, in general, apigenin and naringenin derivatives were not reported by other researchers21,26,41. These observations underscore the need for further investigations into supplements based on Morus alba leaves available on the Polish market.
The quantification of all analysed compounds is presented in Table 3. Predominantly, chlorogenic acid emerged as the dominant compound, displaying a content range of 2.07 to 25.0 mg/100 ml (M4 and L2, respectively), with the highest levels observed in samples derived from a natural collection. The neochlorogenic acid exhibited a content within the range of 0.157–0.611 mg/100 ml, a trend consistent with findings reported by Sánchez-Salcedo et al.38, reaffirming the predominance of phenolic acids over flavonoids, with chlorogenic acid as the primary compound.
Table 3.
Content of individual phenolic compounds in Morus alba L. infusions (mg/100 ml).
| Sample | 3-Caffeoylquinic acid (neochlorogenic acid) | Quercetin- 3,7-O-di-hexoside | Chlorogenic acid | Quercetin- 3-O-rutinoside (rutin) | Quercetin- 3-O- glucoside (isoquercitrin) |
Quercetin- 3-O-(6’’-O-malonyl)-glucoside | Kaempferol- 3-O-glucoside (astragalin) |
Kaempferol 3-O-(6’’-O-malonyl)-glucoside |
|---|---|---|---|---|---|---|---|---|
| Commercial products | ||||||||
| M1 | 0.157 ± 0.014f | 0.122 ± 0.020gh | 2.07 ± 0.234d | 0.307 ± 0.052e | 0.292 ± 0.061 h | 0.135 ± 0.025e | 0.162 ± 0.028f. | 0.099 ± 0.014f. |
| M2 | 0.445 ± 0.052b–d | 0.211 ± 0.021cd | 5.13± 0.715cd | 1.02 ± 0.145e | 0.956 ± 0.130e–g | 0.615 ± 0.101c–e | 0.443 ± 0.046c–f | 0.416 ± 0.047c–e |
| M3 | 0.223 ± 0.025ef | 0.123 ± 0.004gh | 3.22 ± 0.430cd | 0.432 ± 0.050e | 0.512 ± 0.078gh | 0.263 ± 0.038de | 0.230 ± 0.036d–f | 0.176 ± 0.020ef |
| M4 | 0.305 ± 0.052b–f | 0.132± 0.021f–h | 3.47 ± 0.589cd | 0.600 ± 0.163e | 0.478 ± 0.123gh | 0.351 ± 0.102de | 0.291 ± 0.038d–f | 0.296 ± 0.054c–f |
| M5 | 0.455 ± 0.042a–c | 0.176 ± 0.005d–f | 5.19 ± 0.312cd | 1.18 ± 0.079ed | 1.09 ± 0.067d–f | 0.701 ± 0.059c–e | 0.509 ± 0.036b–d | 0.485 ± 0.044 cd |
| M6 | 0.246 ± 0.013ef | 0.133 ± 0.012e–h | 3.17 ± 0.314 | 0.484 ± 0.039e | 0.589 ± 0.033f–h | 0.143 ± 0.014e | 0.287 ± 0.012d–f | 0.114 ± 0.006f. |
| M7 | 0.359 ± 0.024b–e | 0.155 ± 0.006e–g | 4.54 ± 0.331cd | 0.651 ± 0.050e | 1.32 ± 0.034c–e | 0.469 ± 0.041c–e | 0.477 ± 0.021b–e | 0.286 ± 0.026c–f |
| M8 | 0.429 ± 0.010b–d | 0.105 ± 0.011h | 3.64 ± 0.145cd | 0.713 ± 0.033e | 0.787 ± 0.074e–h | 0.275 ± 0.007de | 0.364 ± 0.026c–f | 0.211 ± 0.006d–f |
| M9 | 0.433 ± 0.037b–d | 0.129 ± 0.017gh | 3.51 ± 0.281cd | 0.447 ± 0.009e | 0.692 ± 0.029f–h | 0.164 ± 0.010e | 0.447 ± 0.004c–f | 0.180 ± 0.005ef |
| Raw plant material | ||||||||
| L1 | 0.611 ± 0.122a | 0.247 ± 0.021bc | 16.68 ± 5.835b | 5.06 ± 1.032a | 2.89 ± 0.538b | 1.23 ± 0.978bc | 0.632 ± 0.272bc | 0.476 ± 0.294 cd |
| L2 | 0.289 ± 0.073d–f | 0.274 ± 0.019b | 25.0 ± 2.024a | 3.84 ± 0.326b | 1.71 ± 0.208c | 5.40 ± 0.425a | 0.282 ± 0.037d–f | 2.084 ± 0.182a |
| L3 | 0.293 ± 0.059c–f | 0.162 ± 0.016e–g | 13.4 ± 0.445 | 2.19 ± 0.076c | 0.878 ± 0.028e–g | 1.548 ± 0.053b | 0.170 ± 0.020ef | 0.560 ± 0.017c |
| L4 | 0.377 ± 0.028b–e | 0.154 ± 0.007e–g | 3.72 ± 0.288cd | 0.446 ± 0.039e | 0.747 ± 0.081e–h | 0.147 ± 0.018e | 0.352 ± 0.034c–f | 0.116 ± 0.012f. |
| L5 | 0.459 ± 0.092ab | 0.179 ± 0.018de | 4.68 ± 0.639cd | 0.900 ± 0.122e | 1.61 ± 0.226cd | 0.476 ± 0.066c–e | 0.764 ± 0.115b | 0.339 ± 0.050c–f |
| L6 | 0.430 ± 0.034b–d | 0.330 ± 0.016a | 7.14 ± 0.139c | 1.91 ± 0.149c | 3.69 ± 0.311a | 1.01 ± 0.008b–d | 2.20 ± 0.251a | 0.870 ± 0.058b |
Values in the same row marked with different letters differ significantly (p < 0.05). Content of compound 1 is expressed as caffeic acid equivalent, compounds 2 and 6 as quercetin equivalents and compound 8 as kaempferol equivalent.
Among the glycosides of flavonoids, rutin had the highest content, ranging from 0.307 to 5.06 mg/100 ml, with sample L1 from the natural collection exhibiting the highest concentration. The contents of isoquercitrin and two other quercetin derivatives (quercetin- 3,7-O-di-hexoside and quercetin- 3-O-(6’’-O-malonyl)-glucoside), along with astragalin and a kaempferol 3-O-(6’’-O-malonyl)-glucoside, exhibited diversity within the following ranges: 0.292–3.692, 0.105–0.330, 0.135–5.398, 0.162–2.198, and 0.099–2.084 mg/100 ml, respectively. Notably, glycosides of quercetin were more abundant than those of kaempferol, aligning with the scientific discourse outlined in Sánchez-Salcedo et al.38 and Ju et al.39.
Spectrophotometric techniques were used to assess the total phenolic content and antioxidant activity of white mulberry infusions through two different assays. The results of the TPC, Trolox equivalent antioxidant capacity, and ferric reducing antioxidant power analyses are summarized in Table 4.
Table 4.
Total phenolics content (TPC), Trolox equivalent antioxidant capacity (TEAC) and ferric-reducing antioxidant power (FRAP).
| Sample | TPC | TEAC | FRAP |
|---|---|---|---|
| (mg/100 ml) | (mmol Trolox/100 ml) | (mmol Fe2+/100 ml) | |
| Commercial products | |||
| M1 | 19.0 ± 1.307e | 0.0453 ± 0.000fg | 0.242 ± 0.010ef |
| M2 | 27.2 ± 0.724b–e | 0.0822 ± 0.006c–e | 0.319 ± 0.014b–f |
| M3 | 23.0 ± 1.138de | 0.0472 ± 0.00fg | 0.251 ± 0.010d–f |
| M4 | 18.1 ± 1.137e | 0.0626 ± 0.002ef | 0.291 ± 0.016c–f |
| M5 | 18.1 ± 1.137e | 0.0742 ± 0.001de | 0.310 ± 0.011c–f |
| M6 | 20.9 ± 1.568e | 0.0354 ± 0.026g | 0.202 ± 0.150f |
| M7 | 35.2 ± 0.779b–d | 0.0649 ± 0.005ef | 0.371 ± 0.009b–d |
| M8 | 27.1 ± 0.582b–e | 0.0580 ± 0.002e–g | 0.327 ± 0.008b–e |
| M9 | 35.2 ± 1.704b–d | 0.0700 ± 0.003ef | 0.305 ± 0.005c–f |
| Raw plant material | |||
| L1 | 52.6 ± 1.931a | 0.1470 ± 0.008b | 0.597 ± 0.023a |
| L2 | 49.9 ± 1.481a | 0.1842 ± 0.006a | 0.572 ± 0.019a |
| L3 | 36.8 ± 0.734b | 0.0962 ± 0.012 cd | 0.400 ± 0.012bc |
| L4 | 19.7 ± 1.483e | 0.0605 ± 0.001ef | 0.244 ± 0.004ef |
| L5 | 23.8 ± 1.818c–e | 0.0801 ± 0.007de | 0.336 ± 0.022b–e |
| L6 | 36.2 ± 2.614bc | 0.1071 ± 0.002c | 0.436 ± 0.026b |
The TPC ranged from 18.1 to 52.6 mg (CAE)/100 ml. These findings are comparable to the literature9,38. Those studies express the results as gallic acid equivalent (GAE). It should be noted that CAE delivers slightly higher values when compared with GAE9. shows in case of plants cultivated in Italy that TPC values were higher in case of infusions when compared with hydromethanolic extract. In contrary38, focused on hydromethanolic extraction of the leaves collected from plants originating from Spain and obtained higher results than9. What is more38, shows also that significant differences in the TPC were observed in case of clones of the same specimen. Moreover, the antioxidant activities, as determined by TEAC and FRAP assays, differed across the samples, ranging from 0.0354 to 0.184 mmol TE/100 ml and from 0.202 to 0.597 mmol Fe2+/100 ml, respectively.
Notably, the TEAC and FRAP values obtained in our investigation were relatively lower than those reported in the literature9,38. These discrepancies are likely attributed to following factors. First are differences in the methodological modifications employed in our study compared to the referenced research. Another one are differences between plants used in the study. Not only place of the origin, climatic and soil conditions are essential. Phenotypic factor of the plants cultivated in the same area influences significantly the variability within a chemical profile of a plant.
The majority of the recorded values were notably higher for infusions prepared using leaves sourced from the natural collection, specifically L1, L2, L3, and L6. Conversely, samples L4-L5, which also originated from a natural collection, exhibited lower values that closely resembled those of commercially available products. For instance, the content of rutin (p < 0.001), a recognized potent antioxidant, is an example. Furthermore, the phenolic composition of mulberry leaves can be influenced by various factors, including food processing methods43, such as the drying technique and growth period22. Employing diverse processing methods may result in the accumulation or loss of specific groups of compounds, such as flavonoids, along with alterations in their antioxidant activity. Among the available drying methods, air drying at room temperature has emerged as the most efficient approach for retaining flavonoids in white mulberry leaves, both in terms of preservation and economic feasibility, compared to freeze drying.
Notably, the growth period assumes significance when examining samples from a natural collection, as their growth cycles lack standardization, similar to those from commercially managed orchards. This factor can also influence the phenolic content within infusions prepared from commercially available products or those sourced from a natural collection. Nonetheless, our study conclusively demonstrated that samples derived from natural collections are characterized by a notably high phenolic compound content.
To explore the potential influence of individual compound contents on both the TPC and antioxidant activity of the infusions, we calculated Pearson correlation coefficients (p ≤ 0.05), as presented in Table 5. The rutin content exhibited strong positive correlations with the TPC (R = 0.86), TEAC (R = 0.91), and FRAP (R = 0.94). Similarly, the chlorogenic acid content displayed notably high positive correlation coefficients with the TPC, and the results of the antioxidant assays (R = 0.83, 0.95, and 0.88, respectively) were comparable to those observed for rutin. Conversely, the contents of neochlorogenic acid and astragalin showed non-significant correlations (p≤ 0.05) with the results obtained from spectrophotometric methods. The contents of the remaining compounds exhibited correlations with the TPC, TEAC, and FRAP at similar levels. Additionally, we observed significant correlations between the contents of various phenolic compounds within the samples. However, neochlorogenic acid exhibited a correlation exclusively with the content of isoquercitrin. These strong positive correlations between the results obtained from spectrophotometric methods and the contents of phenolic compounds in mulberry leaves align with the findings of other researchers9. In the present study, similar findings were observed, including a notable correlation between the flavonoid content and phenolic acid content. However, it is worth highlighting that the FRAP assay results were not significantly correlated with the phenolic content. This difference from the present study may be attributed to the reported variance in phenolic fingerprints, as discussed earlier, which likely influenced the outcomes of the referenced study. It is essential to emphasize that the total phenolic content, antioxidant activity, and phenolic compound contents exhibited remarkable similarities among infusions prepared from commercially available samples. In contrast, the outcomes varied significantly for infusions derived from a natural collection.
Table 5.
Correlations between the compounds, total phenolics, TEAC and FRAP. Values of the correlation coefficient (shaded fields) that are statistically significant are shown in italic.
| R/p | 3-Caffeoylquinic acid (neochlorogenic acid) | Quercetin- 3,7-O-di-hexoside | Chlorogenic acid | Quercetin- 3-O-rutinoside (rutin) | Quercetin- 3-O-glucoside (isoquercitrin) |
Quercetin- 3-O-(6’’-O-malonyl)-glucoside | Kaempferol- 3-O-glucoside (astragalin) |
Kaempferol 3-O-(6’’-O-malonyl)-glucoside | Total phenolics content | TEAC | FRAP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-Caffeoylquinic acid (neochlorogenic acid) | 0.129 | 0.542 | 0.099 | 0.021 | 0.867 | 0.122 | 0.944 | 0.144 | 0.158 | 0.067 | |
| Quercetin- 3,7-O-di-hexoside | 0.41 | 0.012 | 0.004 | < 0.001 | 0.022 | 0.003 | 0.003 | 0.012 | 0.001 | 0.002 | |
| Chlorogenic acid | 0.17 | 0.63 | < 0.001 | 0.077 | < 0.001 | 0.976 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Quercetin- 3-O-rutinoside (rutin) | 0.44 | 0.69 | 0.90 | 0.007 | 0.004 | 0.519 | 0.008 | < 0.001 | < 0.001 | < 0,001 | |
| Quercetin- 3-O-glucoside (isoquercitrin) | 0.59 | 0.89 | 0.47 | 0.67 | 0.231 | < 0.001 | 0.083 | 0.011 | 0.008 | 0.002 | |
| Quercetin- 3-O-(6’’-O-malonyl)-glucoside | 0.05 | 0.59 | 0.92 | 0.69 | 0.33 | 0.967 | < 0.001 | 0.007 | < 0.001 | 0.002 | |
| Kaempferol- 3-O-glucoside (astragalin) | 0.42 | 0.72 | 0.01 | 0.18 | 0.84 | − 0.01 | 0.474 | 0.432 | 0.385 | 0.276 | |
| Kaempferol 3-O-(6’’-O-malonyl)-glucoside | 0.02 | 0.71 | 0.86 | 0.66 | 0.46 | 0.97 | 0.20 | 0.011 | < 0.001 | 0.002 | |
| Total phenolics content | 0.40 | 0.63 | 0.83 | 0.86 | 0.64 | 0.67 | 0.22 | 0.64 | < 0.001 | < 0.001 | |
| TEAC | 0.38 | 0.79 | 0.95 | 0.91 | 0.65 | 0.86 | 0.24 | 0.87 | 0.86 | < 0.001 | |
| FRAP | 0.48 | 0.74 | 0.88 | 0.94 | 0.74 | 0.73 | 0.30 | 0.73 | 0.92 | 0.95 |
To obtain a better understanding of the arrangement of the observations within the dataset, we performed principal component analysis. The results of this analysis are presented in Fig. 2. Principal components 1 and 2 (PC1 and PC2) accounted for the majority of the variance (99.24%). In particular, PC1 explained the vast majority of the data structure, representing 92.83% of the total variation. A strong positive correlation emerged between the TPC, chlorogenic acid content, and PC1. Consequently, this correlation delineated the formation of three distinct clusters of observations. In the preceding discussion section, we reported strong, positive Pearson correlation coefficients between the TPC and chlorogenic acid, the dominant compound within white mulberry leaves. This correlation pattern manifested in the arrangement of the observations. The first cluster encompasses two observations, namely, L1 and L2, exhibiting the highest positive values for PC1. Both of these samples are infusions prepared from leaves sourced from a natural collection. The second cluster comprises four samples, namely, M7, lL6, M9, and L3, which display intermediate values for PC1. This cluster groups infusions prepared from both a natural collection and commercially available products. Finally, the third cluster comprises the remaining observations characterized by negative values for PC1. This cluster contains two samples from a natural collection.
Fig. 2.
Principal component analysis plot of the distribution of variables.
Other researchers have also employed PCA to gain a deeper understanding of the interrelationships and patterns among variables connected to Morus alba2,21,38. In the visual distribution depicted by Sánchez-Salcedo et al.38, certain similarities were noted concerning the influence of specific variables, although without a pronounced impact of any single variable. In our study, the TPC and chlorogenic acid content were the variables that exerted the most substantial influence on the distribution of observations, although the number of variables considered differed.
Gai et al.29demonstrated that individual compounds may contribute to the total variance to different degrees when assessing the activity of the entire mixture (infusion, extract, etc.) under polar conditions (TPC, TEAC, FRAP). Discrepancies with the PCA performed in other studies2,21 present a complex challenge for interpretation and rely upon the type and quantity of variables investigated in each study.
It is crucial to recognize that when spectrophotometric methods are employed to measure factors such as antioxidant activity, the occurrence of synergistic, additive, and antagonistic effects among specific molecules during the reaction period must be acknowledged. These effects depend on the ratio of individual antioxidants present in the reaction mixture. Furthermore, when more than two antioxidants are present, the nature of these effects becomes even more complex44, such as in a natural matrix (Morusalba infusion, extract).
Conclusions
Phenolic acids were found to be the most abundant compounds in white mulberry leaf infusions, with two phenolic acids identified, of which chlorogenic acid was the most abundant. In contrast, among the flavonoids, rutin was the most abundant. While phenolic acids displayed greater overall abundance, flavonoids exhibited greater diversity, including four quercetin derivatives and two kaempferol derivatives, within the infusions. Notably, the former four flavonols were present in greater quantities than the latter two.
A trend revealed that samples sourced from a natural collection exhibited the highest phenolic compound contents. This trend was consistently reflected in the results of the TPC, TEAC, and FRAP analyses, which is consistent with a more robust phenolic fingerprint.
Furthermore, the spectroscopic results correlated significantly with the contents of individual compounds, as indicated by strong positive Pearson correlation coefficients. For instance, the TPC exhibited a noteworthy correlation with the dominant compound, which was further confirmed by PCA. This analysis grouped the observations into three distinct clusters, primarily guided by this pair of variables. The first cluster contained samples from a natural collection, the third predominantly included commercially available samples, and the second cluster exhibited a mixed composition.
If the quality of white mulberry leaf infusion is at least partially defined as the levels of dominant antioxidant compounds present in the beverage, our approach successfully segregated the samples with the highest quality. Moreover, our findings underscore the comparability of commercially available products, signalling the need for novel production methods or alternative raw materials. Our findings suggest the possibility of further investigations into white mulberry leaf products available on the Polish market.
Supplementary Information
Acknowledgements
Funded by the Minister of Science under „the Regional Initiative of Excellence Program".
Abbreviations
- ABTS
2,2’-Azinobis(3-ethylbenzothiazoline- 6-sulfonic acid)
- CAE
Catechin equivalent
- FCR
Folin-Ciocâlteu’s reagent
- FRAP
Ferric reducing antioxidant power
- ISSR-PCR
Intersimple sequence repeat polymerase chain reaction technique
- LC-Q-TOF–MS/MS
Liquid chromatography quadrupole time-of-flight tandem mass spectrometry
- PCA
Principal component analysis
- SD
Standard deviation
- TEAC
Trolox equivalent antioxidant capacity
- TPC
Total phenolic content
- TPTZ
2,4,6-Tri(2-pyridyl)-s-triazine
- Trolox
6-Hydroxy- 2,5,7,8-tetramethyl-chroman- 2-carboxylic acid
- RP-HPLC–DAD
Reversed phase high performance liquid chromatography with diode-array detector
Author contributions
Michał Adam Janiak: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Anna Gryn-Rynko: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Katarzyna Sulewska: Investigation, Data curation, Formal analysis, Ryszard Amarowicz: Conceptualization, Methodology, Data curation, Formal analysis, Visualization, Supervision. Kamila Penkacik: Investigation, Data curation, Formal analysis. Radomir Graczyk: Methodology, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Dorota Olszewska-Słonina: Conceptualization, Visualization, Supervision. Michał Stanisław Majewski: Visualization, Supervision. All authors have read and agreed to the published version of the manuscript.
Data availability
The data that support the findings of this study will be available on request from the authors (Michał Adam Janiak, Anna Gryn-Rynko).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in Reference 11. The correct reference is listed here: Herman, M., Janiak, M. A., Sadlik, J. K., Piekoszewski, W. & Amarowicz, R. Iron, zinc, copper, manganese and chromium in green teas, their transfer to extracts and correlations between contents of elements and bioactive compounds. PJFNS. 72(4), 421–429. https://doi.org/10.31883/pjfns/156394 (2022).
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/11/2025
A Correction to this paper has been published: 10.1038/s41598-025-05857-6
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-97223-9.
References
- 1.Yu, Y. et al. Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials. Int. J. Food Prop.21, 1495–1507. 10.1080/10942912.2018.1489833 (2018). [Google Scholar]
- 2.Sánchez-Salcedo, E. M., Amorós, A., Hernández, F. & Martínez, J. J. Physicochemical properties of white (Morus alba) and black (Morus nigra) mulberry leaves a new food supplement. J. Food Nutr. Res.5, 253–261. 10.12691/jfnr-5-4-7 (2017). [Google Scholar]
- 3.Calixto, J. B. The role of natural products in modern drug discovery. Acad. Bras. Cienc.91, e20190105. 10.1590/0001-3765201920190105 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Tran, N., Pham, B. & Le, L. Bioactive compounds in anti-diabetic plants: from herbal medicine to modern drug discovery. Biol.9(9), 252. 10.3390/biology9090252 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltaci, C. et al. Physicochemical properties, antioxidant capacity and sensory acceptability of instant rosehip teas prepared by spray-drying and freeze-drying methods. Pol. J. Food Nutr. Sci. Sciences.74(3), 244–254. 10.31883/pjfns/191929 (2024). [Google Scholar]
- 6.Hardinasinta, G., Mursalim, M., Muhidong, J. & Salengke, S. Effect of ohmic heating on the rheological characteristics and electrical conductivity of mulberry (Morus nigra) puree. PJFNS.71(3), 289–297. 10.31883/pjfns/140151 (2021). [Google Scholar]
- 7.Ta, T. M. N. et al. Effects of mulberry pomace addition and transglutaminase treatment on the quality of pasta enriched with antioxidants and dietary fiber. PJFNS.73(4), 301–310. 10.31883/pjfns/172244 (2023). [Google Scholar]
- 8.Jeong, J. Y. et al. Pancreatic lipase inhibitory constituents from Morus alba leaves and optimization for extraction conditions. BMCL.25(11), 2269–2274. 10.1016/j.bmcl.2015.04.045 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Polumackanycz, M., Wesolowski, M. & Viapiana, A. Morus alba L. and Morus nigra L. leaves as a promising food source of phenolic compounds with antioxidant activity. Plant Foods Hum. Nutr.76, 458–465. 10.1007/s11130-021-00922-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drabińska, N., Nogueira, M., Ciska, E. & Jeleń, H. Effect of drying and broccoli leaves incorporation on the nutritional quality of durum wheat pasta. PJFNS.72(3), 273–285. 10.31883/pjfns/152070 (2022). [Google Scholar]
- 11.Herman, M., Janiak, M. A., Sadlik, J. K., Piekoszewski, W. & Amarowicz, R. Iron, zinc, copper, manganese and chromium in green teas, their transfer to extracts and correlations between contents of elements and bioactive compounds. PJFNS.72(4), 421–429. 10.31883/pjfns/156394 (2022). [Google Scholar]
- 12.Huang, S. S. et al. A comparison of food-grade folium mori extract and 1-Deoxynojirimycin for glycemic control and renal function in streptozotocin-induced diabetic rats. J. Tradit. Complement. Med.10.4103/2225-4110.131639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaipitakwong, T., Numhom, S. & Aramwit, P. Mulberry leaves and their potential effects against cardiometabolic risks: a review of chemical compositions, biological properties and clinical efficacy. Pharm. Biol.56, 109–118. 10.1080/13880209.2018.1424210 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aramwit, P., Petcharat, K. & Supasyndh, O. Efficiacy of mulberry leaf tablets in patients with mild dyslipidemia. Phytother. Res.25, 365–369. 10.1002/ptr.3270 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Jan, B. et al. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Food Res. Int.28, 3909–3921. 10.1016/j.sjbs.2021.03.056 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirvulescu, M. M. et al. Curcumin and a Morus alba extract reduce pro-inflammatory effects of esistin in human endothelial cells. Phytother. Res.25, 1737–1742. 10.1002/ptr.3463 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Dat, N. T. et al. Cytotoxic prenylated flavonoids from Morus alba. Fitoterapia81, 1224–1227. 10.1016/j.fitote.2010.08.006 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Fathy, S. A. et al. The antiproliferative effect of mulberry (Morus alba L.) plant on hepatocarcinoma cell line HepG2. Egypt. J. Med. Hum. Genet.14, 375–382. 10.1016/j.ejmhg.2013.07.001 (2013). [Google Scholar]
- 19.Jeong, J. Y. et al. Characterization of melanogenesis inhibitory constituents of Morus alba leaves and optimization of extraction conditions using response surface methodology. Molecules20, 8730–8741. 10.3390/molecules20058730 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gryn-Rynko, A., Bazylak, G. & Olszewska-Slonina, D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed. Pharmacother.84, 628–636. 10.1016/j.biopha.2016.09.081 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Sánchez-Salcedo, E. M. et al. (Poly)phenolic fingerprint and chemometric analysis of white (Morus alba L.) and black (Morus nigra L.) mulberry leaves by using a non-targeted UHPLC-MS approach. Food Chem.212, 250–255. 10.1016/j.foodchem.2016.05.121 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Hu, L. et al. Flavonoid levels and antioxidant capacity of mulberry leaves: effects of growth period and drying methods. Front. Plant Sci.12, 684974. 10.3389/fpls.2021.684974 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gryn-Rynko, A. et al. The impact of Morus alba L. leaf extract on intestinal ion transport An in vitro study. Biomed. Pharmacother.150, 112939. 10.1016/j.biopha.2022.112939 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Baraniak, J., Kania-Dobrowolska, M. & Kujawski, R. Food supplements in Poland in context of issues related to their safety as food. Herba Polonica.68(3), 36–42. 10.2478/hepo-2022-0015 (2022). [Google Scholar]
- 25.https://www.nik.gov.pl/najnowsze-informacje-o-wynikach-kontroli niekontrolowane-suplementy-diety.html (access: 10.01.2025).
- 26.Dugo, P. et al. Characterization of the polyphenolic fraction of Morus alba leaves extracts by HPLC coupled to a hybrid IT-TOF MS system. J. Sep. Sci.32(21), 3627–3634. 10.1002/jssc.200900348 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Polumackanycz, M., Sledzinski, T., Goyke, E., Wesolowski, M. & Viapiana, A. A comparative study on the phenolic composition and biological activities of Morus alba L. commercial samples. Molecules24(17), 3082. 10.3390/molecules24173082 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gai, F., Karamać, M., Janiak, M. A., Amarowicz, R. & Peiretti, P. G. Sunflower (Helianthus annuus L.) plants at various growth stages subjected to extraction—comparison of the antioxidant activity and phenolic profile. Antioxidants9(6), 535. 10.3390/antiox9060535 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gai, F., Janiak, M. A., Sulewska, K., Peiretti, P. G. & Karamać, M. Phenolic compound profile and antioxidant capacity of flax (Linum usitatissimum L.) harvested at different growth stages. Molecules28(4), 1807. 10.3390/molecules28041807 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peiretti, P. G. et al. Phenolic composition and antioxidant activities of soybean (Glycine max (L.) Merr.) lant during growth cycle. Agron.9(3), 153. 10.3390/agronomy9030153 (2019). [Google Scholar]
- 31.Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med.26, 1231–1237. 10.1016/s0891-5849(98)00315-3 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Benzie, I. F. & Strain, J. J. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurements of total antioxidant power and ascorbic concentration. Methods Enzymol.299, 15–27. 10.1016/s0076-6879(99)99005-5 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Stanisz, A. Easy course of statistic using Statistica PL and medicine examples, 1. In Basic Statistic 532 (StatSoft Polska: Kraków, Poland 2006).
- 34.McDonald, J. H. Handbook of Biological Statistics (2nd ed.). 319 (Sparky House Publishing, 2009).
- 35.Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: Paleontological statistics software package for education and data analysis. Palaeont. Electr.1, 9 (2014). [Google Scholar]
- 36.Granato, D., Calado, V. M. D. A. & Jarvis, B. Observations on the use of statistical methods in food science and technology. Food Res. Int.55, 137–149. 10.1016/j.foodres.2013.10.024 (2014). [Google Scholar]
- 37.Clifford, M. N., Johnston, K. L., Knight, S. & Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem.51(10), 2900–2911. 10.1021/jf026187q (2003). [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Salcedo, E. M., Mena, P., García-Viguera, C., Hernández, F. & Martínez, J. J. (Poly) phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: Their potential for new products rich in phytochemicals. J. Funct. Foods.18, 1039–1046. 10.1016/j.jff.2015.03.053 (2015). [Google Scholar]
- 39.Ju, W. T. et al. Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J. Food Sci. Technol.55, 1789–1796. 10.1007/s13197-018-3093-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, H. X. et al. Identification of anti-melanogenesis constituents from Morus alba L. leaves. Molecules23(10), 2559. 10.3390/molecules23102559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pothinuch, P. & Tongchitpakdee, S. Phenolic analysis for classification of mulberry (Morus spp.) leaves according to cultivar and leaf age. J Food Qual.10.1155/2019/2807690 (2019). [Google Scholar]
- 42.Chan, E. W. C., Wong, S. K., Tangah, J., Inoue, T. & Chan, H. T. Phenolic constituents and anticancer properties of Morus alba (white mulberry) leaves. J. Integr. Med.18(3), 189–195. 10.1016/j.joim.2020.02.006 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Chang S K. How food structure and processing affect the bioavailability of nutrients and antioxidants. Encyclopedia of Food Chemistry (ed. Melton, F. Shahidi, P. Varelis) 158-166 (Elsevier, 2019).
- 44.Slavova-Kazakova, A., Janiak, M. A., Sulewska, K., Kancheva, V. D. & Karamać, M. Synergistic, additive, and antagonistic antioxidant effects in the mixtures of curcumin with (-)-epicatechin and with a green tea fraction containing (-)-epicatechin. Food Chem.360, 129994. 10.1016/j.foodchem.2021.129994 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study will be available on request from the authors (Michał Adam Janiak, Anna Gryn-Rynko).