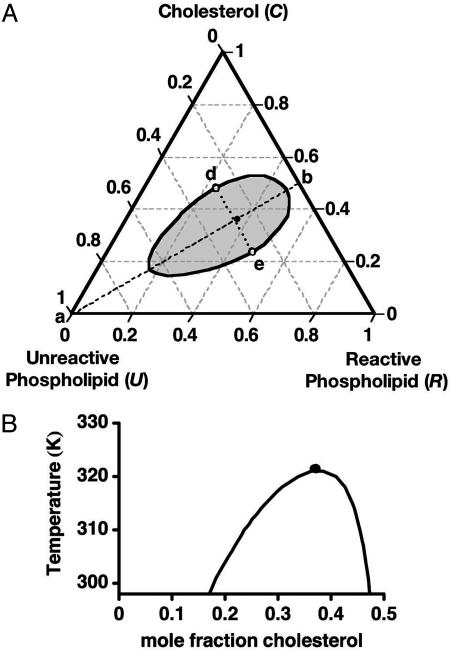
Fig. 1.
Calculated phase diagrams for a ternary mixture of cholesterol and two phospholipids. One phospholipid (R) reacts with cholesterol to form a 1:1 complex. The complex (X) is only partially miscible with the second phospholipid (U). (A) Isothermal ternary diagram at 298 K for equilibrium constant K = 142 and binary U–X critical temperature T0c = 484 K. Along the stoichiometric line a–b the initial concentrations of the reactants are equal. The open circles at d and e are critical points at this temperature. The filled circle close to the line d–e gives the ternary critical composition at 323 K. (B) Pseudobinary phase diagram for compositions along the line a–b in A. The heat of reaction is taken to be –9.6 kcal/mol. The filled circle again denotes the ternary critical point.